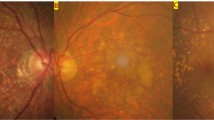
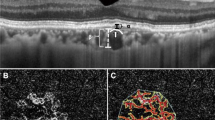
Abstract
Background
Pachychoroid, or the structural and functional abnormalities of the choroid, is one of the most important causes of exudative maculopathies. The purpose of this article is to review the current definitions of pachychoroid and their potential consequences.
Summary of findings
Most publications are from Asian countries. Although no consensus diagnosis has been reached, pachychoroid is defined by thickened choroid and choroidal vascular hyperpermeability, pachyvessels with inner choroidal attenuation; it is closely linked to pachydrusen. Although some studies suggest choroidal congestion may play a role in its pathogenesis, the exact causes of this condition are still unknown. Pachychoroid is associated with exudative maculopathies including central serous chorioretinopathy, pachychoroid neovasculopathy and polypoidal choroidal vasculopathy (PCV). It is widely accepted that macular neovascular membranes may develop secondary to pachychoroid. Recent clinical observations illustrate the importance of pachychoroid in the etiology of macular neovascularization including neovascular age-related macular degeneration (nAMD).
Conclusion
Pachychoroid is an important cause of exudative maculopathies. Both drusen and pachychoroid are increasingly recognized as important causes of macular neovascularization, and eyes formally categorized as typical nAMD or PCV can be further sub-categorized based on the presence or absence of pachychoroid and drusen. There is a need to develop a consensus definition, which will greatly enhance our understanding of pachychoroid and facilitate the development of individual interventions in pachychoroid diseases.










Similar content being viewed by others
References
Spaide RF, Hall L, Haas A, Campeas L, Yannuzzi LA, Fisher YL, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16:203–13.
Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986;9:37–41.
Guyer DR. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057.
Sasahara M, Tsujikawa A, Musashi K, Gotoh N, Otani A, Mandai M, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006;142:601–7.e1.
Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5.
Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–73.
Jirarattanasopa P, Ooto S, Nakata I, Tsujikawa A, Yamashiro K, Oishi A, et al. Choroidal thickness, vascular hyperpermeability, and complement factor h in age-related macular degeneration and polypoidal choroidal vasculopathy. Investig Opthalmol Vis Sci. 2012;53:3663.
Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S. Relationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeability. Am J Ophthalmol. 2013;155:305–13.e1.
Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33:1659–72.
Siedlecki J, Schworm B, Priglinger SG. The pachychoroid disease spectrum—and the need for a uniform classification system. Ophthalmol Retina. 2019;3:1013–5.
Iida T, Hagimura N, Takahashi K, Muraoka K. Study of choroidal vascular lesions in bullous retinal detachment by indocyanine green angiography. J Jpn Ophthalmol Soc. 1995;99:945–54 (in Japanese).
Yanagi Y, Ting DSW, Ng WY, Lee SY, Mathur R, Chan CM, et al. Choroidal vascular hyperpermeability as a predictor of treatment response for polypoidal choroidal vasculopathy. Retina. 2018;38:1509–17.
Miyake M, Tsujikawa A, Yamashiro K, Ooto S, Oishi A, Tamura H, et al. Choroidal neovascularization in eyes with choroidal vascular hyperpermeability. Investig Opthalmology Vis Sci. 2014;55:3223.
Fung AT, Yannuzzi LA, Freund K. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina. 2012;32:1829–37.
Nomura Y, Takahashi H, Tan X, Obata R, Yanagi Y. Widespread choroidal thickening and abnormal midperipheral fundus autofluorescence characterize exudative age-related macular degeneration with choroidal vascular hyperpermeability. Clin Ophthalmol. 2015;9:297–304.
Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015;35:1–9.
Chang Y-C, Cheng C-K. Difference between pachychoroid and nonpachychoroid polypoidal choroidal vasculopathy and their response to anti-vascular endothelial growth factor therapy. Retina. 2019. https://doi.org/10.1097/iae.0000000000002583.
Miyake M, Ooto S, Yamashiro K, Takahashi A, Yoshikawa M, Akagi-Kurashige Y, et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep. 2015;5:16204.
Nakayama M, Keino H, Okada AA, Watanabe T, Taki W, Inoue M, et al. Enhanced depth imaging optical coherence tomography of the choroid in Vogt–Koyanagi–Harada disease. Retina. 2012;32:2061–9.
Spaide RF, Goldbaum M, Wong DWK, Tang KC, Iida T. Serous detachment of the retina. Retina. 2003;23:820–46.
Lee M, Lee H, Kim HC, Chung H. Changes in stromal and luminal areas of the choroid in pachychoroid diseases: insights into the pathophysiology of pachychoroid diseases. Investig Ophthalmol Vis Sci. 2018;59:4896–908.
Dansingani KK, Balaratnasingam C, Naysan J, Freund KB. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina. 2016;36:499–516.
Baek J, Lee JH, Jung BJ, Kook L, Lee WK. Morphologic features of large choroidal vessel layer: age-related macular degeneration, polypoidal choroidal vasculopathy, and central serous chorioretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2018;256:2309–17.
Hiroe T, Kishi S. Dilatation of asymmetric vortex vein in central serous chorioretinopathy. Ophthalmol Retina. 2018;2:152–61.
Matsumoto H, Kishi S, Mukai R, Akiyama H. Remodeling of macular vortex veins in pachychoroid neovasculopathy. Sci Rep. 2019;9:14689.
Mori K. Asymmetry of choroidal venous vascular patterns in the human eye. Ophthalmology. 2004;111:507–12.
Daizumoto E, Mitamura Y, Sano H, Akaiwa K, Niki M, Yamanaka C, et al. Changes of choroidal structure after intravitreal aflibercept therapy for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2017;101:56–61.
Yun C, Huh J, Ahn SM, Lee B, Kim JT, Hwang SY, et al. Choriocapillaris flow features and choroidal vasculature in the fellow eyes of patients with acute central serous chorioretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:57–70.
Rochepeau C, Kodjikian L, Garcia M-AA, Mathis T. Optical coherence tomography angiography quantitative assessment of choriocapillaris blood flow in central serous chorioretinopathy. Am J Ophthalmol. 2018;194:26–34.
Gal-Or O, Dansingani KK, Sebrow D, Dolz-Marco R, Freund KB. Inner choroidal flow signal attenuation in pachychoroid disease : optical coherence tomography angiography. Retina. 2018;38:1984–92.
Sakurada Y, Fragiotta S, Leong BCS, Parikh R, Hussnain SA, Freund KB. Relationship between choroidal vascular hyperpermeability, choriocapillaris flow density, and choroidal thickness in eyes with pachychoroid pigment epitheliopathy. Retina. 2019. https://doi.org/10.1097/iae.0000000000002635.
Baek J, Kook L, Lee WK. Choriocapillaris flow impairments in association with pachyvessel in early stages of pachychoroid. Sci Rep. 2019;9:5565.
Demirel S, Değirmenci MFK, Batıoğlu F, Özmert E. Evaluation of the choroidal features in pachychoroid spectrum diseases by optical coherence tomography and optical coherence tomography angiography. Eur J Ophthalmol. 2019. https://doi.org/10.1177/1120672119887095.
Nichole J, Mitchell P, Younan C, Burlutsky G, Cheng C, Cheung CMG, et al. Ethnic variation in early age-related macular degeneration lesions between white Australians and Singaporean asians. Investig Ophthalmol Vis Sci. 2014;55:4421–9.
Lee J, Kim M, Lee CS, Kim SS, Koh HJ, Lee SC, et al. Drusen subtypes and choroidal characteristics in asian eyes with typical neovascular age-related macular degeneration. Retina. 2020;40:490–8.
Lee J, Byeon SH. Prevalence and clinical characteristics of pachydrusen in polypoidal choroidal vasculopathy: multimodal image study. Retina. 2019;39:670–8.
Matsumoto H, Mukai R, Morimoto M, Tokui S, Kishi S, Akiyama H. Clinical characteristics of pachydrusen in central serous chorioretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:1127–32.
Baek J, Lee JH, Chung B, Lee K, Lee WK. Choroidal morphology under pachydrusen. Clin Exp Ophthalmol. 2019;47:498–504.
Spaide RF. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina. 2018;38:708–16.
Cheung CMG, Gan A, Yanagi Y, Wong TY, Spaide R. Association between choroidal thickness and drusen subtypes in age-related macular degeneration. Ophthalmol Retina. 2018;2:1196–205.
Singh S, Chakurkar R, Goud A, Rasheed M, Vupparaboina K, Chhablani J. Pachydrusen in polypoidal choroidal vasculopathy in an Indian cohort. Indian J Ophthalmol. 2019;67:1121–6.
Singh SR, Oli A, Mohan S, Goud A, Rasheed MA, Vupparaboina KK, et al. Pachydrusen in Indian population: a hospital-based study. Indian J Ophthalmol. 2019;67:371–5.
Fukuda Y, Sakurada Y, Yoneyama S, Kikushima W, Sugiyama A, Matsubara M, et al. Clinical and genetic characteristics of pachydrusen in patients with exudative age-related macular degeneration. Sci Rep. 2019;9:11906.
Lee J, Choi S, Lee CS, Kim M, Kim SS, Koh HJ, et al. Neovascularization in fellow eye of unilateral neovascular age-related macular degeneration according to different drusen types. Am J Ophthalmol. 2019;208:103–10.
Ersoz MG, Arf S, Hocaoglu M, Sayman Muslubas I, Karacorlu M. Indocyanine green angiography of pachychoroid pigment epitheliopathy. Retina. 2018;38:1668–74.
Ersoz MG, Karacorlu M, Arf S, Hocaoglu M, Sayman Muslubas I. Pachychoroid pigment epitheliopathy in fellow eyes of patients with unilateral central serous chorioretinopathy. Br J Ophthalmol. 2017;102:473–8.
Karacorlu M, Ersoz MG, Arf S, Hocaoglu M, Sayman Muslubas I. Long-term follow-up of pachychoroid pigment epitheliopathy and lesion characteristics. Graefe’s Arch Clin Exp Ophthalmol. 2018;256:2319–26.
Ersoz MG, Karacorlu M, Arf S, Hocaoglu M, Sayman Muslubas I. Outer nuclear layer thinning in pachychoroid pigment epitheliopathy. Retina. 2018;38:957–61.
Lee JH, Kim JY, Jung BJ, Lee WK. Focal disruptions in ellipsoid zone and interdigitation zone on spectral-domain optical coherence tomography in pachychoroid pigment epitheliopathy. Retina. 2019;39:1562–70.
Takahashi A, Ooto S, Yamashiro K, Tamura H, Oishi A, Miyata M, et al. Pachychoroid geographic atrophy. Ophthalmol Retina. 2018;2:295–305.
Yanagi Y, Mohla A, Lee WK, Lee SY, Mathur R, Chan CM, et al. Prevalence and risk factors for nonexudative neovascularization in fellow eyes of patients with unilateral age-related macular degeneration and polypoidal choroidal vasculopathy. Investig Opthalmology Vis Sci. 2017;58:3488–95.
Kim K, Kim JM, Kim DG, Yu S-Y, Kim ES. Five-year follow-up of unaffected fellow eyes in patients with polypoidal choroidal vasculopathy. Ophthalmologica. 2019. https://doi.org/10.1159/000501212.
Baek J, Lee JH, Lee WK. Retinoschisis in eyes with pachychoroid and retinal pigment epithelial atrophy. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:1863–71.
Hariri A, Heussen FM, Nittala MG, Sadda SVR. Optical coherence tomographic correlates of angiographic subtypes of occult choroidal neovascularization. Investig Ophthalmol Vis Sci. 2013;54:8020–6.
Sato T, Kishi S, Watanabe G, Matsumoto H, Mukai R. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina. 2007;27:589–94.
Sheth J, Anantharaman G, Chandra S, Sivaprasad S. “Double-layer sign” on spectral domain optical coherence tomography in pachychoroid spectrum disease. Indian J Ophthalmol. 2018;66:1796–801.
Pichi F, Morara M, Veronese C, Ciardella AP. The overlapping spectrum of flat irregular pigment epithelial detachment investigated by optical coherence tomography angiography. Int Ophthalmol. 2018;38:975–83.
Hwang H, Kim JY, Kim KT, Chae JB, Kim DY. Flat irregular pigment epithelium detachment in central serous chorioretinopathy. Retina. 2019. https://doi.org/10.1097/iae.0000000000002662.
Dansingani KK, Balaratnasingam C, Klufas MA, Sarraf D, Freund KB. Optical coherence tomography angiography of shallow irregular pigment epithelial detachments in pachychoroid spectrum disease. Am J Ophthalmol. 2015;160:1243–54.e2.
Carnevali A, Capuano V, Sacconi R, Querques L, Marchese A, Rabiolo A, et al. OCT angiography of treatment-naïve quiescent choroidal neovascularization in pachychoroid neovasculopathy. Ophthalmol Retina. 2017;1:328–32.
Forte R, Coscas F, Serra R, Cabral D, Colantuono D, Souied EH. Long-term follow-up of quiescent choroidal neovascularisation associated with age-related macular degeneration or pachychoroid disease. Br J Ophthalmol. 2019. https://doi.org/10.1136/bjophthalmol-2019-315189.
Terao N, Koizumi H, Kojima K, Yamagishi T, Nagata K, Kitazawa K, et al. Association of upregulated angiogenic cytokines with choroidal abnormalities in chronic central serous chorioretinopathy. Investig Opthalmol Vis Sci. 2018;59:5924–31.
Hata M, Yamashiro K, Ooto S, Oishi A, Tamura H, Miyata M, et al. Intraocular vascular endothelial growth factor levels in pachychoroid neovasculopathy and neovascular age-related macular degeneration. Investig Ophthalmol Vis Sci. 2017;58:292–8.
Terao N, Koizumi H, Kojima K, Yamagishi T, Yamamoto Y, Yoshii K, et al. Distinct aqueous humour cytokine profiles of patients with pachychoroid neovasculopathy and neovascular age-related macular degeneration. Sci Rep. 2018;8:10520.
Azuma K, Tan X, Asano S, Shimizu K, Ogawa A, Inoue T, et al. The association of choroidal structure and its response to anti-VEGF treatment with the short-time outcome in pachychoroid neovasculopathy. PLoS One. 2019;14:e0212055.
Cho HJ, Jung SH, Cho S, Han JO, Park S, Kim JW. Intravitreal anti-vascular endothelial growth factor treatment for pachychoroid neovasculopathy. J Ocul Pharmacol Ther. 2019;35:174–81.
Jung BJ, Kim JY, Lee JH, Baek J, Lee K, Lee WK. Intravitreal aflibercept and ranibizumab for pachychoroid neovasculopathy. Sci Rep. 2019;9:2055.
Matsumoto H, Hiroe T, Morimoto M, Mimura K, Ito A, Akiyama H. Efficacy of treat-and-extend regimen with aflibercept for pachychoroid neovasculopathy and Type 1 neovascular age-related macular degeneration. Jpn J Ophthalmol. 2018;62:144–50.
Roy R, Saurabh K, Shah D, Goel S. Treatment outcomes of pachychoroid neovasculopathy with photodynamic therapy and anti-vascular endothelial growth factor. Indian J Ophthalmol. 2019;67:1678.
Baek J, Lee JH, Jeon S, Lee WK. Choroidal morphology and short-term outcomes of combination photodynamic therapy in polypoidal choroidal vasculopathy. Eye. 2019;33:419–27.
Padrón-Pérez N, Arias L, Rubio M, Lorenzo D, García-Bru P, Català-Mora J, et al. Changes in choroidal thickness after intravitreal injection of anti-vascular endothelial growth factor in pachychoroid neovasculopathy. Investig Ophthalmol Vis Sci. 2018;59:1119–24.
Hara C, Wakabayashi T, Toyama H, Fukushima Y, Sayanagi K, Sato S, et al. Characteristics of patients with neovascular age-related macular degeneration who are non-responders to intravitreal aflibercept. Br J Ophthalmol. 2019;103:623–9.
Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen SJ, Chen Y, Freund KB, et al. Polypoidal choroidal vasculopathy. Ophthalmology. 2018;125:708–24.
Ueta T, Obata R, Inoue Y, Iriyama A, Takahashi H, Yamaguchi T, et al. Background comparison of typical age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2009;116:2400–6.
Ueta T, Iriyama A, Francis J, Takahashi H, Adachi T, Obata R, et al. Development of typical age-related macular degeneration and polypoidal choroidal vasculopathy in fellow eyes of Japanese patients with exudative age-related macular degeneration. Am J Ophthalmol. 2008;146:96–101.e2.
Chung H, Byeon SH, Freund KB. Focal choroidal excavation and its association with pachychoroid spectrum disorders. Retina. 2017;37:199–221.
Lee JH, Park H-YL, Baek J, Lee WK. Alterations of the lamina cribrosa are associated with peripapillary retinoschisis in glaucoma and pachychoroid spectrum disease. Ophthalmology. 2016;123:2066–76.
Phasukkijwatana N, Freund KB, Dolz-Marco R, Al-Sheikh M, Keane PA, Egan CA, et al. Peripapillary pachychoroid syndrome. Retina. 2018;38:1652–67.
Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid disease. Eye. 2019;33:14–33.
Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39:367–74.
Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51.
Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144:15–22.e2.
Yoshimura N. Age-related Macular Degeneration in the Japanese. Nihon Ganka Gakkai Zasshi. 2016;120:163–88 (discussion 189, in Japanese).
Takahashi K, Ishibashi T, Ogur Y, Yuzawa M. Classification and diagnostic criteria of age-related macular degeneration. Nihon Ganka Gakkai Zasshi. 2008;112:1076–84 (in Japanese).
Tan CS, Ngo WK, Chen JP, Tan NW, Lim TH. EVEREST study report 2: imaging and grading protocol, and baseline characteristics of a randomised controlled trial of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99:624–8.
Yannuzzi LA, Negrão S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter J, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–34.
Freund KB, Ho IV, Barbazetto IA, Koizumi H, Laud K, Ferrara D, et al. Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina. 2008;28:201–11.
Wong CW, Yanagi Y, Lee WK, Ogura Y, Yeo I, Wong TY, et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016;53:107–39.
Hageman G. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32.
Yanagi Y, Foo VHX, Yoshida A. Asian age-related macular degeneration: from basic science research perspective. Eye. 2019;33:34–49.
Yuzawa M. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2005;89:602–7.
Tanaka K, Mori R, Kawamura A, Nakashizuka H, Wakatsuki Y, Yuzawa M. Comparison of OCT angiography and indocyanine green angiographic findings with subtypes of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2017;101:51–5.
Coscas G, Lupidi M, Coscas F, Benjelloun F, Zerbib J, Dirani A, et al. Toward a specific classification of polypoidal choroidal vasculopathy: idiopathic disease or subtype of age-related macular degeneration. Investig Ophthalmol Vis Sci. 2015;56:3187–95.
Jang JW, Kim JM, Kang SW, Kim SJ, Bae K, Kim KT. Typical polypoidal choroidal vasculopathy and polypoidal choroidal neovascularization. Retina. 2019;39:1995–2003.
Hata M, Tagawa M, Oishi A, Kawashima Y, Nakata I, Akagi-Kurashige Y, et al. Efficacy of photodynamic therapy for polypoidal choroidal vasculopathy associated with and without pachychoroid phenotypes. Ophthalmol Retina. 2019;3:1016–25.
Lee WK, Baek J, Dansingani KK, Lee JH, Freund KB. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina. 2016;36:S73–82.
Hosoda Y, Yoshikawa M, Miyake M, Tabara Y, Ahn J, Woo SJ, et al. CFH and VIPR2 as susceptibility loci in choroidal thickness and pachychoroid disease central serous chorioretinopathy. Proc Natl Acad Sci. 2018;115:6261–6.
Lehmann M, Bousquet E, Beydoun T, Behar-Cohen F. Pachychoroid. Retina. 2015;35:10–6.
Hosoda Y, Yamashiro K, Miyake M, Ooto S, Oishi A, Miyata M, et al. Predictive genes for the prognosis of central serous chorioretinopathy. Ophthalmol Retina. 2019;3:985–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Y. Yanagi, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Organizer: Akitaka Tsujikawa, MD
Corresponding Author: Yasuo Yanagi
About this article
Cite this article
Yanagi, Y. Pachychoroid disease: a new perspective on exudative maculopathy. Jpn J Ophthalmol 64, 323–337 (2020). https://doi.org/10.1007/s10384-020-00740-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00740-5