Abstract
Ophidiomyces ophidiicola (Oo) is one of the most relevant fungal pathogens for snakes. It is the etiological agent of ophidiomycosis, an emerging disease causing dysecdysis, skin abnormalities, crusting cutaneous lesions, and ulcerations. Despite this major tegumentary “tropism”, Oo infection can be systemic and it is capable of inducing visceral lesions. Moreover, ophidiomycosis may lead to abnormalities of reproductive physiology, hunting behavior, and thermoregulation, thus increasing the risks of sublethal effects and predation on affected snakes. Oo seems horizontally transmitted and can induce postnatal mortality. This article reviews published data on Oo detection and infection in all snake species in countries around the world and categorizes these data using new classification parameters. The presence of this fungus has been recorded in 11 states (considering the USA as a whole); however, in four states, the mycosis has only been reported in snakes held in captivity. Detection and/or infection of Oo has been ascertained in 62 snake species, divided into nine families. The taxa have been categorized with diagnostic criteria in order to report, for each species, the highest rank of categorization resulting from all cases. Therefore, 20 species have been included within the class “Ophidiomycosis and Oo shedder”, 11 within “Ophidiomycosis”, 16 in “Apparent ophidiomycosis”, and 15 within “Ophidiomyces ophidiicola present”. We also discuss the significance and limits of case classifications and Oo’s impact on wild populations, and we suggest methods for preliminary surveillance. Standardized methods, interdisciplinary studies, and cooperation between various research institutions may facilitate further Oo screening studies, elucidate the unclear aspects of the disease, and protect ophidiofauna from this emerging threat at the global level.
Similar content being viewed by others
Introduction
Fungal pathogens are considered an emerging concern for wildlife (Fisher et al. 2012). They are usually capable of infecting multiple hosts and can be environmentally resistant (Reynolds et al. 2017). For example, mycotic diseases caused by Batrachochytrium dendrobatidis, B. salamandrivorans, and Pseudogymnoascus destructans are often fatal for amphibians and chiroptera (Langwig et al. 2016; Stegen et al. 2017; O’Hanlon et al. 2018). Although there are fewer specific data regarding reptile populations, these animals could also be menaced by infectious diseases. Furthermore, at least one-fifth of the evaluated reptile species all over the globe are considered endangered (Böhm et al. 2013). Emerging pathogens, particularly onygenalean fungi, are a matter of concern for reptiles worldwide (Paré and Conley 2020), both for veterinary practice (Piquet et al. 2018; Paré et al. 2020) and herpetofauna (Allain and Duffus 2019; Peterson et al. 2020). Within Onygenales, fungi affecting reptiles are present in genera such as Ophidiomyces, Parananizziopsis, Emydomyces, and Nannizziopsis. These fungi have various similar morphological and histological features but have been recently discovered or split into different species via molecular analyses (Sigler et al. 2013; Stchigel et al. 2013; Woodburn et al. 2019). Nannizziopsis and Parananizziopsis are genera that affect different Reptilia orders, while Emydomyces testavorans only affects chelonians and Ophidiomyces ophidiicola (Oo) specifically affects ophidian hosts. Among these pathogens, Oo is currently the most studied and very likely has the most impact on natural populations of reptiles.
History, taxonomy, and phylogenesis of Oo
Since the discovery of Oo, the taxonomy of this fungus has changed many times. Other closely related onygenalean fungi, also belonging to the former Chrysosporium anamorph of Nannizziopsis vriesii (CANV) complex, have undergone similar taxonomic changes (Paré and Sigler 2016). Furthermore, the origin of Oo remains unclear since there are scattered cases and/or detections in almost every ecozone around the world. Whether or not Oo is now ubiquitous in terms of distribution, this fungus has probably been present for an indefinitely long time outside its native range. Indeed, a very recent molecular-based study suggests that the fungus has been introduced to North America on multiple occasions since the 18th century (see Ladner et al. 2022).
Here we present, in chronological order, reported case studies in which Oo has been detected (prospectively or retrospectively) in order to highlight and clarify the taxonomic changes that have occurred and to show the reported distribution of this organism. In each case presented below, the fungus detected or isolated has since been identified or reclassified as Oo.
A specimen of Cemophora coccinea collected in Florida in 1945, a Crotalus horridus from Wisconsin (1958), and a Pantherophis spiloides collected in Tennessee in 1973, all showing cutaneous lesions, were preserved in 70% ethanol and stored at the University of Wisconsin Zoological Museum (Lorch et al. 2021). From these specimens, in 2021 Lorch and colleagues retrospectively detected Oo with molecular and histopathological analysis.
In 1985, researchers isolated a fungus and observed hyphae in histology from the subcutaneous lesions of a captive Python regius from England (Sigler et al. 2013).
Similarly, a fungus was isolated from a specimen of Pantherophis guttatus that presented with a subcutaneous nodule on the tail in 1986, in New York, USA (Sigler et al. 2013). This was confirmed with histological sections exhibiting hyphae and arthroconidia.
In 1990, a fatal dermatomycosis in three Boiga irregularis wild captured from Guam and housed in Maryland (USA) was diagnosed by histopathology and isolation of CANV, which was identified according to growth characteristics and morphology (Nichols et al. 1999; Sigler et al. 2013).
In the last part of the 1990s, a captive Thamnophis in Germany died after showing signs of mycosis in the skin, lung, and liver ascribed to Chrysosporium queenslandicum after fungal culture and histopathology (Vissiennon et al. 1999; Sigler et al. 2013).
Two different cultures of CANV were isolated from snakes with skin lesions found in Australia: from Acrochordus sp. found in Queensland in 2003 (Sigler et al. 2013) and from Hoplocephalus bungaroides found in Victoria in 2010 (McLelland et al. 2010; Sigler et al. 2013).
A free-ranging Pantherophis obsoletus (considered P. alleghaniensis by Lorch et al. 2016a) captured in Georgia (USA) in the first half of the 2000s and held for 4 years while regularly used in educational exhibitions showed multifocal facial granulomas containing fungal hyphae and segmented arthroconidiating hyphae (Rajeev et al. 2009; Lorch et al. 2016a). After isolation and morphological, cultural, and molecular characterization, the fungus was classified as Chrysosporium ophiodiicola (Guarro, D. A. Sutton, Wickes, and Rajeev, sp. nov. (Rajeev et al. 2009).
Starting with a few animals with dermatological lesions and mortality in 2006, a severe fungal disease was thought to be one of the main causes implicated in the New Hampshire (USA) Crotalus horridus population decline (Clark et al. 2011).
Since 2008, a population of Sistrurus catenatus catenatus in Illinois (USA) has started to show signs of Chrysosporium ophiodiicola–associated disease linked with mortality (Allender et al. 2011).
A nomenclatural novelty was published by Sigler (2013), in which Chrysosporium ophiodiicola was reassigned to the new genus Ophidiomyces [Ophidiomyces ophiodiicola (Guarro, Deanna A. Sutton, Wickes & Rajeev) Sigler, Hambleton & Paré, comb.nov.; Basionym: Chrysosporium ophiodiicola], placed phylogenetically within the family Onygenaceae. This publication was integrated into another from Sigler and collaborators (2013), in which the CANV complex was taxonomically reassessed and a number of isolates–several of them coming from some of the above-mentioned cases–were molecularly characterized and retrospectively reclassified as O. ophiodiicola, within onygenacean fungi.
Almost simultaneously in 2013, Stchigel and colleagues solved the taxonomic position of a few other Chrysosporium pathogenic to reptiles, proposing new species and the family of Nannizziopsiaceae. However, O. ophiodiicola (as C. ophiodiicola) was placed in Incertae sedis, between Nannizziopsiaceae and Onygenaceae but phylogenetically closer to the latter (Stchigel et al. 2013). The entire genoma of an Oo isolate was sequenced by Ohkura et al. (2017).
According to the NCBI taxonomy database (Schoch et al. 2020), Index Fungorum (Kirk 2020), and MycoBank (Robert et al. 2013), the currently valid species name is Ophidiomyces ophidiicola (Guarro, Deanna A. Sutton, Wickes, & Rajeev) Sigler et al. 2013. The name Ophidiomyces ophiodiicola is considered an orthographic variant and refers to a name that does not follow standard rules of orthography.
The NCBI taxonomy database (Schoch et al. 2020), Index Fungorum (Kirk 2020), MycoBank (Robert et al. 2013), UNITE (Nilsson et al. 2019), CoL/GBIF (GBIF Secretariat 2019), and BOLD (Ratnasingham and Hebert 2007) all currently list O. ophidiicola as part of the family Onygenaceae.
To elucidate the phylogenetic relationship between Oo isolates, sequences derived from the ITS, ACT, and TEF genes of the fungus were aligned, concatenated, and submitted to maximum likelihood estimation and Bayesian analysis (Franklinos et al. 2017; Sun et al. 2021). These analyses have yielded a phylogenetic tree supporting the existence of three clades:
-
Clade 1 (named European clade), derived from the UK and Czech Republic isolates (Franklinos et al. 2017);
-
Clade 2 (named North American clade), consisting of isolates from the USA (Franklinos et al. 2017) and two isolates from a Naja atra individual native to Taiwan (Sun et al. 2021);
-
Clade 3 derives from two isolates from a wild Dinodon rufozonatum in Taiwan (Sun et al. 2021) and a 1985 isolate from the skin lesions of a Python regius held in captivity in the UK (Franklinos et al. 2017; Sun et al. 2021).
A recent characterization of some isolates highlighted that all strains from wild USA snakes belong to Clade 2, that seems to originate itself from the older European Clade 1 (Ladner et al. 2022). Moreover, Oo strains from Taiwan, as well as, from captive snakes from Australia, Europe and North America were grouped within the outgroup Clade 3 or within different lineages of Clade 2 also found in Eastern USA wild snakes, suggesting a spillover via pet trade (Ladner et al. 2022).
Common name of the disease
The terminology snake fungal disease (SFD) appeared for the first time in 2013, when there was a lack of clear evidence of disease association with Oo (Sleeman 2013). The causality of the disease and the direct link with the pathogen were later confirmed by the fulfilment of Koch’s postulates via experimental infection of captive-bred Pantherophis guttatus with a cultured fungal isolate (Lorch et al. 2015). In this case, Lorch and colleagues (2015) proposed to use the term SFD to refer only to Oo infections. The denomination SFD is considered by some authors a simplistic name that should be rejected and replaced with Ophidiomycosis, Oo infection, or Oo mycosis (Paré et al. 2020). This suggestion is also in line with the fact that other fungal pathogens occur in snakes and some of them are also former members of CANV complex, such as Paranannizziopsis spp. (Sigler et al. 2013).
Fungal properties in the environment, fungal cultures, and tissues
Various Oo traits, such as the ability to be cultivated on different decaying (autoclaved) organisms, led to the suggestion that it is a saprobe fungus (Allender et al. 2015c). Its suitability to different environments is supported by its capability of growing in a broad range of pH and temperatures in vitro, tolerating air dryness (matric-induced water stress) and most natural sulfur compounds, and its ability to use various complex carbon and nitrogen sources (Allender et al. 2015c). The microorganism can grow on soil medium (TWRA 2017) and can be detected in natural soil (Walker et al. 2019; Campbell et al. 2021). However, Campbell and colleagues (2021) showed that Oo was able to grow only in sterilized soil and not in soils with an active microbial community. Oo growth seems to be inhibited by metabolites produced by other fungal taxa and via general suppression by whole microbial communities in soil (Campbell et al. 2021). Moreover, Campbell et al. (2021) revealed that the prevalence of Oo detection was significantly higher in hibernacula soils compared with topsoils. This information is consistent with the potential role of hibernacula as an environmental reservoir, where the pathogen can persist in its viable propagule: the conidium (spore).
Propagules are a reproductive state of an organism represented in this ascomycete by asexual (anamorph) conidia (Rajeev et al. 2009; Sigler et al. 2013). In Oo cultures, they can be divided into aleurioconidia and arthroconidia (Rajeev et al. 2009; Sigler 2013; Sigler et al. 2013). Aleuriospores grow on short stalks on the sides of the hyphae from which they detach by rhexolytic dehiscence (Fig. 1a). Arthrospores, in contrast, are generated by fragmentation of pre-existing fertile hyphae (schizolytic dehiscence–Fig. 1b). Aleuroconidia are cylindrical to clavate, 2.5–8.3 μm long, and 1.5–2.9 μm wide (Fig. 1a; Sigler et al. 2013; Ohkura et al. 2016). Arthroconidia have a longer cylindrical form with slightly obtuse to truncate ends, ranging from 3 to 12.5 μm in length and 1.5–3.5 μm in width (Fig. 1b–Sigler et al. 2013). In Oo cultures, vegetative (main) hyphae are septate, branched, thin walled, narrow, sometimes with racquet-shaped mycelium, and 1.5–2.5 μm wide (Rajeev et al. 2009; Sigler 2013; Sigler et al. 2013). Undulating branches of hyphae are sometimes seen lateral to the main hyphae (Fig. 1b insert–Sigler et al. 2013; Ohkura et al. 2016). This is very characteristic for most of the former members of the CANV complex (Paré and Sigler 2016). The fertile main hyphae and the lateral undulating hyphal branches can differentiate into chains of adjacent arthroconidia (Sigler et al. 2013; Ohkura et al. 2016).
Microscopic (a and b) and macroscopic (c and d) features of Ophidiomyces ophidiicola (Oo) cultures. a Microscopic morphology Oo aleurioconidia (arrows) growing on short stalks on the sides of the hyphae from which they will detach by rhexolytic dehiscence (isolate UAMH number 10769). b Cylindrical fission arthroconidia created by schizolytic dehiscence (fragmentation) of fertile hyphae (isolate UAMH number 11295). b (Insert) undulate hyphae growing laterally to the main hyphae (isolate UAMH number 11295). c and d Colonies of Oo shown on PDA at 30 °C after 21 days of incubation (c zonate, powdery colony–isolate UAMH number 10769; d flat, dense, velvety colony–isolate UAMH number 11295). Photos courtesy of Lynne Sigler and UAMH Centre for Global Microfungal Biodiversity
Oo incubation on potato dextrose agar (PDA) at 25–30 °C for 14–21 days results in colonies with a diameter varying from 31 to 60 mm (Rajeev et al. 2009; Sigler et al. 2013; Ohkura et al. 2016; Sun et al. 2021) and its growth tends to be inhibited at 35 °C (Sigler et al. 2013; Sun et al. 2021). Oo growth is limited at 15 °C (5-mm diameter after 14-day incubation–Rajeev et al. 2009); however, the fungus is able to survive freezing (Paré and Sigler 2016). Colonies appear whitish-to-light yellow (Fig. 1c, d; Rajeev et al. 2009; Sigler et al. 2013). At 25 °C, the colonies are flat, dense, and powdery-to-velvety (e.g., Fig. 1d; Rajeev et al. 2009; Sun et al. 2021), and at 30 °C they frequently appear zonate (e.g., Fig. 1c–Sigler et al. 2013).
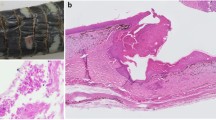
To date, only the form of hyphae or arthroconidia has been recognized in histological sections of Oo. Histologically, the hyphae are usually intralesional and have parallel walls, a diameter up to 5 μm, transverse septa, and frequent acute-angle branching (Fig. 2a, c, d–Baker et al. 2019). In contrast, arthroconidia in histological section are approximately 2 × 4 μm, cylindrical, and are found intralesionally in fission formation (separating from fertile hyphae) and/or in compact clusters (Fig. 2b, b insert–Baker et al. 2019). The latter aggregates/clusters are called arthroconidial tufts and are sometimes found at the air-tissue interface at the surface of the lesion or within granulomas (Paré and Sigler 2016). Arthroconidia are also produced in vivo in the skin surface of hosts and the presence of arthroconidial tufts (arthroconidiation) at this location is considered by Paré and Sigler (2016) to be “practically pathognomonic for infection with one of these fungi” (i.e., former members of the CANV complex).
Histological features of Ophidiomyces ophidiicola (Oo) in cutaneous tissues. a Histological section of scale tip showing Oo hyphae. Note the hyphal aggregate in the ventral region of the scale, likely developing into an arthroconidial tuft through fission and arthroconidiation. PAS staining. b Arthroconidia (arrows) located at the air-tissue interface. PAS staining of a 5 µm section. b (Insert) Arthroconidia (arrows) in 5-µm section stained with Grocott. c Oo hyaline hyphae showing parallel walls, transverse septa, and acute-angle branching. PAS staining. d Intralesional Oo hyphae in Grocott-stained section
The air-tissue interfaces at the skin or within internal organs, such as bronchi (Dolinski et al. 2014), are the most common localization for aggregate-forming arthrospores (Fig. 2a; Dolinski et al. 2014; Haynes et al. 2021; Last et al. 2016; Baker et al. 2019). This is consistent with the intrinsic infective propagule nature of dermatophyte arthroconidia and their propensity to be dispersed in the environment (Patel et al. 2017). To the authors’ knowledge, no Oo aleuroconidia have been described in histological sections.
Pathogenesis of ophidiomycosis
It is very likely that natural Oo infection of healthy snakes occurs via contact with symptomatic/asymptomatic carriers or contaminated substratum. Although interruption of the continuity of the epidermis (e.g., wounds, abrasions, or ulcerations) is not necessary to provoke fungal invasion and subsequent macroscopic lesions, it seems to increase the frequency of such signs (Lorch et al. 2015). The dermatomycosis can involve individual scales (e.g., Bickinese 2009) or extensively affect the surface of dorsal, ventral, and head scales (e.g., Mckenzie et al. 2020a).
Results of different experimental infections have confirmed that the fungus is able to infect via conidium, regardless which cutaneous/para-cutaneous route is chosen (Allender et al. 2015a; Lorch et al. 2015; McKenzie et al. 2020b). Inoculation of conidia via topical application on nasolabial pits (Allender et al. 2015a), topical bandage on different parts of the tegument, with or without skin abrasion (Lorch et al. 2015), or subcutaneous injection (McKenzie et al. 2020b) resulted in mild-to-severe effects (McKenzie et al. 2020b).
The survival rate of exposed snakes from the above-mentioned experimental studies (Allender et al. 2015a; Lorch et al. 2015; McKenzie et al. 2020b) varies from 0 to 87%, mostly due to different experimental designs (e.g., inoculation route and amount of conidia inoculated). For example, while in the Lorch et al. (2015) study, the disease did not progress toward life-threatening stages, resulting in no “natural” deaths, McKenzie and colleagues (2020b) also challenged infected snakes with brumation, leading to a higher mortality rate. These data suggest that brumation and lower temperatures may have a negative impact on survival in snakes with fungal infections.
The skin lesions that develop during experimental cutaneous Oo exposures include regional swelling, scale edema and discoloration/yellowing, lesion enlargement and coalescence, vesicle formation, and serocellular crusting (Lorch et al. 2015; McKenzie et al. 2020b). Crusts can detach revealing erosions and ulcers (Lorch et al. 2016a; Meier et al. 2018). Similarly, abnormal scales may progressively turn into dermal granulomas that subsequently become eroded or ulcerated (Baker et al. 2019). Other cutaneous signs that are also seen in naturally infected animals (e.g., Franklinos et al. 2017; Pohly 2020) include dysecdysis, wrinkling, desquamation, and necrotic/brownish scales (Fig. 3–e.g., Lorch et al. 2016a; Meier et al. 2018; Sun et al. 2021).
Cutaneous gross signs consistent with Ophidiomyces ophidiicola mycosis commonly observed in Natrix helvetica positive at molecular detection (Apparent Ophidiomycosis). (a) Crusty lesions, dysecdysis, scales distortion, wrinkling and discolouration on the head region. (b) Scales margin erosion on the gular region. (c) Focal lesion with crusty and necrotic aspect. Note the presence of surrounding displaced or oedemic scales. (d) Crusting and marginal erosion of ventral scales. (e) Healed dorsal scales (previously affected by crusty lesions) showing scars and wrinkling. (f) Healed ventral scales (previously affected by crusts/erosions) showing scars and wrinkling. Photo credits: (a, b, c, d) Courtesy of Steven J. R. Allain and Becki Lawson; (e, f) Courtesy of Grégoire Meier
When the inflammation is mild, cutaneous lesions usually show only heterophilic infiltration, while in moderate cases of inflammation, the white blood cell population is mixed, including lymphocytes and macrophages. More severely inflamed lesions may also involve the underlying skeletal muscles (see McKenzie et al. 2020a, b).
Increased frequency of ecdysis has been described in some Oo cases in free-ranging snakes (e.g., Tetzlaff et al. 2015; Meier et al. 2018), although ecdysis was not significantly different in snakes exposed via subcutaneous inoculation (below the dermis) under controlled conditions (McKenzie et al. 2020b). Furthermore, the moulting process can apparently clear fungal infections restricted to the superficial epidermis (Lorch et al. 2015). However, it is unlikely that the shedding process can remove the fungus when it reaches the α-layer of the epidermis, as in this case it has already crossed the mesos layer, an important barrier preventing water loss (Jacobson 2007), and has become a deeper infection.
Once past the epidermis, the fungus can cause multifocal dermal or hypodermal granulomas that contain a central area of necrosis and are often visible externally as nodules (Allender et al. 2011; Ohkura et al. 2016). This pattern suggests a possible hematogenous spread within the dermis (McKenzie et al. 2020b).
Some subcutaneous fungal granulomas can reach the coelomic cavity, the coelomic fat pad, and the kidneys, by spreading through connective tissues and epaxial skeletal muscles (Robertson et al. 2016; Steeil et al. 2018a, b).
Non-tegumentary granuloma formation associated with naturally occurring or experimental infections can involve several structures (disseminated ophidiomycosis), including air sacs, bronchi, lungs, trachea, esophagus, stomach, mesentery, gingiva, salivary glands, eyes, coelomic fat, ovaries, kidneys, liver, and spleen (Allender et al. 2011; Dolinski et al. 2014; McKenzie et al. 2020b; Pohly 2020; Robertson et al. 2016; Steeil et al 2018a; Vissiennon et al. 1999). In the latter cases, the presence of fungal elements within the granulomas was not always reported. This may be due to liquefaction of the necrotic cores or low sensitivity of the dyes used, but could also indicate an absence of Ophidiomyces infection. For this reason, the association between cutaneous and visceral infection remains unclear (Pohly 2020). However, Oo’s ability to cause systemic infections is supported by the presence of fungal elements consistent with this pathogen in the liver (McKenzie et al. 2020b; Vissiennon et al. 1999), serosal vessels adjacent to gastroenteric tract (McLelland et al. 2010), and lungs (Dolinski et al. 2014; Robertson et al. 2016; Vissiennon et al. 1999). Furthermore, some disseminated cases have been attributed to mycotic septicemia or fungal emboli (Vissiennon et al. 1999; McLelland et al. 2010; Robertson et al. 2016) and a case of fungal invasion into the hepatic vessels associated with thrombosis has been documented with histopathology (Vissiennon et al. 1999).
Nevertheless, despite the data suggesting hematogenous spread (e.g., McKenzie et al. 2020b), the pathogenesis of systemic Ophidiomyces invasion, and an eventual adaptive immune response of the host, remains uncharacterized (Pohly 2020).
A cohort study by Pohly (2020) using 23 wild-caught naturally infected Nerodia sipedon insularis found histopathological evidence of subclinical disease (early (acute) or resolving (chronic) inflammation and infection) in close to 50% of cases. These data suggest that subclinical lesions may be almost as abundant as macroscopic ones, raising the problem of false negatives when Oo is only present at the subepidermal or visceral level.
In the same study by Pohly (2020), the most prevalent comorbidity associated with ophidiomycosis was a considerable decrease in visceral adipose tissue, suggesting that the mortality due to this fungal infection might be caused by a secondary and continuous negative energy balance, as commonly occurs in chronic diseases that stimulate catabolism via pro-inflammatory cytokines (Wang and Ye 2015).
Postnatal mortality due to rapid ophidiomycosis development has been reported in different taxa of housed wild snakes from North America (Britton et al. 2019; Stengle et al. 2019). In these cases, Oo was likely transmitted by direct contact from the dams to the offspring during or after birth, in both viviparous and oviparous species. Clinical signs in neonates can be subtle or absent, unlike in the parents (Britton et al. 2019).
Skin microbiota and ophidiomycosis
On the one hand, the microbiota in vertebrates is fundamental in defence against pathogens as it can interact with noxious microorganisms through competition for space and resources, release of antifungal compounds/antagonists, modulation of the host immune response, and/or stabilization of the microbial community to increase defence mechanisms (Harris et al. 2006; Lauer et al. 2007; Bletz et al. 2013; Park et al. 2014; Smeekens et al. 2014; Woodhams et al. 2014). On the other hand, microbial diversity can be disrupted by an invading pathogen (Barman et al. 2008; Round and Mazmanian 2009). For example, B. dendrobatidis (the cause of amphibian chytridiomycosis) induces changes in the skin microbiome in wild frogs (Jani and Briggs 2014) and B. salamandrivorans induces changes in the skin microbiome in fire salamanders during experimental infection (Bletz et al. 2018). Although the protective function of cutaneous symbiotic microbes has been widely studied in amphibian diseases, data regarding snakes and Ophidiomyces infection is relatively scarce.
Allender and colleagues (2018) showed that the bacterial and fungal microflora in a population of wild Sistrurus catenatus were disrupted in snakes with a swab positive for Oo. The pathogen, when present, was the dominant fungal species and the community structure of bacterial species was different in the Oo-positive samples (Allender et al. 2018): for example, the genera Janthinobacterium and Serratia were significantly more abundant, whereas Cellulosimicrobium and Rhodococcus were significantly reduced. Moreover, Oo was also present in skin locations distant from the lesions, suggesting a reorganization of the microbiota across the whole tegument (Allender et al. 2018).
The microbial skin assemblage of snakes and related interactions with Oo have also been spatially characterized by Walker et al. (2019). Their study showed that the microflora of snake skin was partially distinct from the environmental microbial communities and that the ophidian species was strongly predictive of the skin microbiota at various spatial extensions. Oo was a strong predictor of snake-skin microbial assemblages and its load was associated with different bacterial taxa (Walker et al. 2019). This result is also supported by the fact that 16 bacterial strains from the skin microflora of Coluber constrictor and Crotalus horridus individuals showed inhibitory effects against Oo (Hill et al. 2018), consequently altering the composition of microbial communities when present.
Physiological and behavioral changes linked to Oo infection
Ophidiomyces infection has not only physical costs but also affects the behavior of ophidians in the wild. Generally, snakes are secretive species and the equilibrium between their activities, such as foraging, thermoregulation, and predator avoidance, depends on various aspects related to the species and physiological status. Conditions impairing health, such as ophidiomycosis, may influence the activities carried out by infected serpentes.
Using different methods, it has been shown that infected Natricidae and Viperidae seem more sedentary compared with uninfected individuals (Tetzlaff et al. 2017; McKenzie et al. 2021). Mycosis can influence the visibility of an individual and can be translated into more or less time spent on pursuing an activity. The exposure to a visual encounter was higher in infected individuals of two species of water snakes (Natricidae-McKenzie et al. 2021) but was lower in infected Viperidae (Tetzlaff et al. 2017) compared with uninfected ophidians. This contrast can be explained by the different methods and environments used by different species to forage (e.g., pursuit vs. ambush predation) or variations in the season and temperature during the different surveys.
Temperature has a fundamental role during pathological conditions in reptiles, for example, in snakes that show a behavioral fever response by raising their preferred body temperature through active thermoregulation when challenged with a pyrogen or pyrogenic pathogen (Burns et al. 1996; Zimmerman et al. 2010). When Pantherophis guttatus snakes are immunochallenged with lipopolysaccharide (LPS), a pyrogen that activates the immune system, their predator-avoidance sheltering behavior is deprioritized in favor of thermoregulation outside the shelter (Todd et al. 2016). Behavioral fever has been anecdotally observed during late autumn (Clark et al. 2011; Tetzlaff et al. 2017) and winter months (McBride et al. 2015) in wild animals affected by, or showing signs of, Oo infection. This behavioral response during colder seasons may improve the immune response by obtaining a better cell-mediated response raising temperature to the preferred optimal zone and, synergistically, could slow fungal growth by subjecting Oo to unfavorable higher temperatures. However, as shown in the lab, behavioral fever might have negative consequences due to predation within infected populations (see Sperry et al. 2021).
Ophidiomycosis appears to also influence reproduction in a sublethal manner. The disease resulted in suppression of the hypothalamo-pituitary–gonadal axis in infected S. miliarius females (Lind et al. 2018a). The hormones produced by this axis promote spermatogenesis and vitellogenesis in different seasons (summer and spring, respectively). During these periods, affected snakes have significantly lower levels of testosterone and estradiol compared with healthy conspecifics (Lind et al. 2018a). Although a direct link between infection and possible reproductive impairment has not yet been found, these results, along with the strong negative correlation between infection severity and body condition index (BCI), suggest that Ophidiomycosis might reduce individual fitness and silently affect mating success.
Body weight and snout-vent-length (SVL) are not strong predictors of Ophidiomycosis (see Chandler et al. 2019; Long et al. 2019; McKenzie et al. 2020b). However, calculating a BCI from these measurements may be helpful to compare groups of individuals within a population (see McCoy et al. 2017; Dillon 2020). Although there are many ways to calculate BCIs (e.g., ratio and residual indices), most methods generally predict energy reserves, usually in the form of fat (Labocha et al. 2014). Some Oo-infected individuals have an increased resting metabolic rate, likely associated with mounting an immune response (Agugliaro et al. 2020). This is consistent with the higher energetic costs faced by affected individuals who are often found with decreased visceral adipose tissue at necropsy (Pohly 2020) or with significantly reduced BCIs (McCoy et al. 2017; Lind et al. 2018b, c). Moreover, an increased concentration of circulating glucocorticoids–indicating an increased stress response–found in free-ranging symptomatic individuals (Lind et al. 2018c) can contribute to this decrease in fat reserves (Pohly 2020). The infected individuals may need an increased body temperature/behavioral fever (Agugliaro et al. 2020) and may have an ongoing positive feedback loop of declining condition (Lind et al. 2018c). In this way, they could face a compromised ability to predate and fulfil their basic energy intake requirements (Lorch et al. 2015), which, in turn, may worsen their physical condition and lead to silent death. Nonetheless, one study reported that BCIs and fitness did not significantly differentiate between Oo qPCR negative and positive individuals in a specific population (Dillon 2020). This highlights that the response to the pathogen likely differs among species due to different environments, climates, and seasonal dynamics.
Ophidiomycosis appears to have a certain seasonality, in which the overwintering period, temperatures, and hibernacula potentially play key roles in the manifestation and transmission of the disease.
In ectotherms, abiotic factors, such as temperature, are crucial variables that force the animal to balance the energetic cost of its activities. Immune system efficiency is also influenced by external factors and intrinsic physiological variables, such as the amount of energy reserves that can be spent on its maintenance. For a reptile, the immunocompetence required to tackle an infection is highly dependent on thermoregulation of the body temperature (Todd et al. 2016; Origgi and Tecilla 2020). Therefore, the temperature variations that occur during seasonal shifts may lead a snake to down-regulate immune function (McCoy et al. 2017), especially when energetic reserves are limited and cannot be addressed to defend against pathogens such as Oo. McCoy and colleagues (2017) found that Ophidiomycosis severity scores in non-hibernating Sistrurus miliarius individuals of both sexes in Central Florida peaked when atmospheric temperature was lower (mostly in February). This can be explained by the increased costs of thermoregulation compromising the efficiency of the immune system. In the same species at the same location, Lind et al. (2018c) showed that plasma corticosterone was higher with more severe infections, but was also higher in winter and autumn before the exacerbation of clinical signs. The hypothesis formulated by Lind and colleagues (2018c) is highly plausible: an increased stress response, due to seasonal stressors such as low temperatures and lower resource acquisition, acts synergistically with an increasing need to allocate resources (e.g., reproduction, growth, and immune function) to establish a vicious cycle that drives a seasonal pattern of disease. Furthermore, this positive feedback loop, acting season over season, may increase host susceptibility, and therefore, exacerbate Ophidiomycosis severity and lead to the deteriorating conditions observed in the population through winter (Lind et al. 2018c).
In brumating snake populations, overwintering may have a pivotal role in the manifestation of Ophidiomycosis and survival of the snakes. Individuals have been anecdotally observed to more commonly show signs of disease and poor conditions just after emerging from brumation or during winter months, when they should be hibernating (Clark et al. 2011; McBride et al. 2015; Guthrie et al. 2016; Lorch et al. 2016a). The higher prevalence of Oo and increased detection of clinical signs after the brumation period, as well as a lower prevalence during the active season, was confirmed by Dillon (2020) in Pantherophis vulpinus from Ontario, Canada. Additionally, from the same study, four out of six snakes tracked during overwintering only showed lesions after brumation (Dillon 2020). In KY, the prevalence of pathogens and the presence of lesions in different species of wild snakes follow the same trend, being higher in spring and lower toward autumn (McKenzie et al. 2019). This may occur because low temperatures do not permit snakes to mount an effective immune response or to increase the frequency of shedding (to prevent the fungus from reaching deeper layers of the tegument), or because the pathogen may have higher virulence during certain seasonal variations (McCoy et al. 2017). Similarly, results from McKenzie and colleagues (2020b) showed that more than half of exposed Pantherophis guttatus died during brumation and that all the inoculated ophidians housed in a room simulating spring (19–24 °C) died in the post-brumation period.
For ophidians that brumate, hibernacula may have an important role in pathogen transmission. Transmission during overwintering (i.e., in the shared hibernacula) is credible because snakes that were negative for Oo prior to brumation emerged with the disease (Long et al. 2019; Dillon 2020). In the study by McKenzie and colleagues (2020b), one out of 12 non-inoculated snakes that brumated together with inoculated animals showed positive qPCR results and characteristic inflammatory skin lesions, but no fungal evidence nor gross lesions (case classified as Oo present) suggesting horizontal transmission by direct contact or contamination from the environment. Even though this pattern was not observed in the other 11 snakes, the authors could not rule out whether there was a clearing of infection before the death of the individual or a contamination without infection (McKenzie et al. 2020b). However, it is plausible that Oo transmission in hibernacula is more driven by direct contact between animals than by contamination via shared dens, since soil microbiota is known to suppress the pathogen in the environment (Campbell et al. 2021).
Ophidiomyces ophidiicola impact on snake populations
Although ophidiomycosis is now considered a wide threat to snake populations, the specific effect on free-ranging snakes is poorly known (McKenzie et al. 2021). Many studies focused on the detection of Oo in single individuals and, therefore, our understanding of the real impact of this disease on natural populations is far from complete (Allender et al. 2015c).
In the USA, where most studies on ophidiomycosis have been conducted, several examples show Oo’s potential impact on natural populations. A population of Crotalus horridus suffered a severe decline in 2006 and also showed clinical signs consistent with ophidiomycosis (Clark et al. 2011). Similar effects were observed in a population of Sistrurus catenatus that showed more than 90% mortality of individuals in which the fungus was detected (Allender et al. 2018). An observed mortality event in 2009 in a population of Nerodia sipedon insularis in Lake Erie was linked to the detection of Oo (Lorch et al. 2016a). Despite a previous positive trend, the population declined by an estimated 18% the year following the outbreak (Lorch et al. 2016a). Chandler et al. (2019) registered a concerning Oo prevalence (≥ 50%) in Drymarchon couperi populations, a species that has already declined across much of its range (USFWS 2018; Chandler et al. 2019).
Controversially, McKenzie et al. (2021) reported a high percentage of snakes apparently affected by ophidiomycosis in assessed populations (37.9% of Regina septemvittata; 20.5% of N. sipedon), without identifying the effects of Oo infection on their survival. Likewise, Dillon (2020) reported that Ophidiomycosis (Oo detected in a snake with clinical signs consistent with the disease–apparent Ophidiomycosis) has no direct effects on the fitness of a population of Pantherophis vulpinus in Morpeth, Ontario, Canada. Ophidians with ophidiomycosis were not more likely to die and did not exhibit drastically different movement patterns compared with uninfected snakes (Dillon 2020). However, infected snakes may have a higher risk of predation and ophidians that tested positive by real-time PCR had larger home ranges than snakes that tested negative (Dillon 2020).
Beyond the North American continent, the number of published studies is low. For example, in Europe, the impacts of ophidiomycosis remain unknown due to the lack of health surveillance and long-term monitoring data (Böhm et al. 2013; Franklinos et al. 2017).
Standardized approaches
Standardized methods are essential to harmonize results and tackle an emergent infectious disease that may threaten ophidian species all over the world. In approximately a decade of targeted research, different diagnostic approaches and scores have been used by different researchers.
Diagnostics
Molecular biology methods, such as conventional or quantitative PCR, are the fastest and most reliable method to assess the presence of fungal DNA.
Different sets of primers for the molecular detection of Oo via quantitative or conventional PCR assays have been established:
-
Bohuski et al. (2015) designed primers targeting internal transcribed spacer region 2 (ITS2) and intergenic spacer region (IGS) within the ribosomal RNA (rRNA) gene of Oo;
-
Allender et al. (2015b) designed primers targeting internal transcribed spacer region 1 (ITS1) within the fungal rRNA gene;
-
Lorch et al. (2021) designed primers targeting mitochondrial NADH dehydrogenase subunit 1 (nad1) of Oo.
Fungal DNA can be extracted from various samples. Dry swabs represent the most used method, although wet swabs have also been used (see McKenzie et al. 2019). It is highly recommended to resample the snakes by swabbing multiple times with different applicators because the percentage of qPCR false negatives on material collected with a single swab is remarkably high (> 70%–Hileman et al. 2018). The ophidians should be swabbed along the entire length of the body several times using mild pressure, and, when symptomatic, lesions should be additionally swabbed using moderate pressure (see Baker et al. 2019). Swabs from ophidians with cutaneous clinical signs are more likely to test positive than those from asymptomatic individuals (Hileman et al. 2018; Long et al. 2019). However, asymptomatic animals may have an undetected subepidermal or visceral Oo infection (Pohly 2020). Almost full agreement of fungal copies/ng DNA was found in the qPCR results from swabs, tissue biopsies, and exuviae, suggesting that each of these samples is reliable and allows fungal detection with molecular methods (Baker et al. 2019; Dibadj et al. 2021). Moults can be homogenized and analyzed via qPCR, sometimes highlighting false negatives resulting from previous swabs (e.g., McKenzie et al. 2020b).
Fungal culture is necessary for Oo isolation. Inoculations of tissue samples, such as skin, exuviae, or cutaneous lesions (e.g., Glorioso et al. 2016), on culture media enriched with antibiotics are required for this method (Baker et al 2019). Skin/lesion swabs are often considered not useful for this diagnostic task (Davy et al. 2021).
The following media are suitable for inoculation and incubation of samples to isolate Oo:
-
DTM (dermatophyte test medium): 30 °C for 20 days (Lorch et al. 2015);
-
SDA (sabaroud dextrose agar): room temperature (or 22–25 °C) for 10–20 days (Bohuski et al. 2015; Dolinski et al. 2014; Allender et al. 2015c; Last et al. 2016; Rzadkowska et al. 2016);
-
PDA (potato dextrose agar): 30–35 °C for 21 days (Sigler et al. 2013);
-
ICG (inhibitory mould agar with chloramphenicol and gentamicin): 25 °C, followed by sub-cultivation on PDA for morphological characterization (Sun et al. 2021).
The morphological traits used to identify Oo in fungal culture are described in “Fungal properties in the environment, fungal cultures and tissues” section and in the cited literature. This method has some disadvantages (Pohly 2020): the complexity of identifying the isolates based on morphologic characteristics, the slow growth of Oo, the difficulty of isolating Oo from small samples, and the growth of other commensal fungi in the media. Nevertheless, fungal culture seems necessary for the extraction of fungal genomic DNA to use for identification, such as 18S rRNA ITS DNA sequencing (Dolinski et al. 2014), and molecular phylogenetic analysis (Franklinos et al. 2017; Sun et al. 2021).
Histopathology has a pivotal role in confirmation of Ophidiomycosis. Skin tissues from necropsies or biopsies are usually used for this task. After fixation, embedding, sectioning, and dewaxing, sections on slides are routinely stained with hematoxylin eosin (HE). Although HE is a useful dye to analyze microscopic lesions and characteristics, it might not highlight fungal hyphae and arthroconidia. For this purpose, other histochemical stains, including silver stains such as Gomori’s (Grisnik et al. 2018; McKenzie et al. 2020a, b), Grocott-Gomori’s (McBride et al. 2015; Ohkura et al. 2016), Grocott’s (Allender et al. 2011; Lorch et al. 2021), or Jones’ (Sun et al. 2021) methenamine silver stains or periodic acid–Schiff (e.g., Last et al. 2016; Lorch et al. 2021; Sun et al. 2021) have been used to characterize the morphology of fungal elements (Fig. 2–see “Discussion and future perspectives” section for further information).
Detection in the field
Clinical signs seem to be a strong predictor of Oo presence during investigation in the field, at least from spring to summer in some locations of Eastern USA (McKenzie et al. 2019; Fuchs et al. 2020).
Ultraviolet fluorescence has proved to be effective as a non-invasive and field-applicable screening tool for Ophidiomycosis (Vivirito et al. 2021), as it has in the past for other emerging fungal diseases, such as white-nose syndrome in bats (Turner et al. 2014). This technique highlights skin lesions in snakes exposed to UV light (365 nm) and, although it seems effective only on snakes with apparent ophidiomycosis, offers the advantage of being fast, reliable, affordable, and useful in the preliminary phase of the screening process, in particular, to identify individuals and skin areas for further diagnostic testing (Vivirito et al. 2021). Further studies, including stricter diagnostic criteria, should be conducted to obtain a more accurate validation of UV light as a diagnostic method for Ophidiomyces because this technique was only compared and validated with qPCR results and not with histological analysis (Vivirito et al. 2021).
Environmental DNA (eDNA), a highly sensitive method for species detection, is rapid and potentially cost-effective (Ficetola et al. 2008). It has been useful in monitoring fungal pathogens in freshwater systems directly from water samples (Strand et al. 2014) and it is sometimes more effective than conventional methods (Wittwer et al. 2018). Baker et al. (2020) tested eDNA for the first time as a survey method to simultaneously identify the DNA of S. catenatus and Ophidiomyces using water samples from over-wintering refugia. Despite local abundance of snakes and fungus at the sites investigated (Allender et al. 2016), they only detected S. catenatus DNA in few samples and did not detect Ophidiomyces. These results suggest that eDNA may not offer more advantages compared with other sampling methods (Baker et al. 2020). Many environmental factors may have adversely affected fungal detection in this study and, therefore, other studies are needed to evaluate the effectiveness of this technique for monitoring Ophidiomyces (Baker et al. 2020).
Case classification
Parsing cases of Oo detection and infection into specific classes are necessary to assess the susceptibility of different ophidian hosts.
The Canadian Wildlife Health Cooperative (CWHC) threat assessment defines suspected and confirmed cases (CWHC 2017), outlined later by Davy and colleagues (2021). Briefly, criteria provided for diagnosis of ophidiomycosis are the following (CWHC 2017; Davy et al. 2021):
-
(D.i) only molecular or culture-based detection of Oo allows the “detected” diagnosis;
-
(D.ii) the presence of gross signs with (D.ii.i) molecular/culture-based detection of Oo or (D.ii.ii) histological confirmation of hyphae consistent with Oo will classify the case as “suspected Ophidiomycosis”;
-
(D.iii) molecular/culture-based detection of Oo and histological confirmation of hyphae consistent with Oo will lead to “Ophidiomycosis” diagnosis.
Likewise, Baker and colleagues (2019) proposed the following diagnostic criteria:
-
(B.i) “Ophidiomyces present” when a positive molecular/culture-based detection is not accompanied by gross or histological alteration consistent with Oo infection;
-
(B.ii) “possible Ophidiomycosis” when (B.ii.i) histology demonstrates fungal hyphae consistent with Oo infection without arthroconidia or (B.ii.ii) when there are gross lesions but PCR/culture was not performed, was equivocal, or negative, or histopathology showed no fungal elements in lesions;
-
(B.iii) “apparent Ophidiomycosis” when (B.iii.i) there are gross (cutaneous) clinical signs and positive molecular/culture-based detection but histopathology was not performed or (B.iii.ii) histopathology is consistent with Oo infection (only hyphae without arthroconidia) and there is a positive molecular/culture-based detection;
-
(B.iv) “confirmed Ophidiomycosis” is the diagnosis if histopathology highlights intralesional hyaline fungal hyphae and arthroconidia and the PCR or culture is positive (no relevance is given to macroscopic signs consistent with Oo infection).
While these criteria are very similar, there is a significant difference: Baker et al. (2019) require the intralesional presence of arthroconidia to “confirm” the diagnosis of ophidiomycosis.
It is fundamental to adopt a consistent, standardized approach to this classification in order to harmonize all future investigations.
Scoring systems
Various scoring systems have been established to harmonize results within single studies. Baker and colleagues (2019) have published a system that scores the severity of macroscopic infections. It includes the type, location, number, and size of tegument lesions and gives a result ranging from 0 to 12. Interpretation of the results and categorization into minor, moderate, or severe infections should be specified using real observations in a determined species (Baker et al. 2019).
A scoring system for microscopic cutaneous lesion severity has been devised by McKenzie and collaborators (2020a). It considers the histological features of a tissue, including inflammation, necrosis, and erosion, resulting in a score ranging from 0 to 8 that leads to the assignment of a histologic grade, 0 (absent), I (mild), II (moderate), or III (severe), to a skin section (McKenzie et al. 2020a).
To harmonize anatomopathological results from a cohort study, Pohly (2020) used a standardized method to detect and classify the severity of cutaneous and systemic lesions starting from the above-mentioned scoring systems. Each snake was divided into 12 regions including head, cloaca, tail, and nine equal sections from the base of the head to the cloaca. For each region, the macroscopic external lesions were localized, uniquely identified, and used to calculate the infection severity score of the whole carcass according to Baker et al. (2019). After necropsy, visceral adipose bodies were classified as adequate or minimal and internal organs, the whole head, and a circumferential section from each region were fixed with 10% neutral-buffered formalin (Pohly 2020). Routine HE staining permitted sections from each region to be histopathologically investigated for fungal presence and graded (0–3) for microscopic lesions, following McKenzie et al. (2020a). The circumferential sections allowed Pohly (2020) to also analyze osseous tissues. Summing the grade of each of the 12 sections, the whole body cutaneous severity grade could reach a maximum of 36 (Pohly 2020). Moreover, anatomopathological examination of visceral organs was performed (Pohly 2020). Although this systematic mapping protocol might need time and resources, it can be a valuable approach to investigate the disease in pilot studies or in small populations and to detect subclinical infections.
Materials and methods
The peer reviewed scientific literature, conference papers, abstracts, student theses, books, and posters on ophidiomycosis sensu lato were screened from inception to June 30, 2021 through PubMed and Embase bibliographic databases (Fig. 4). The following keyword string was used for the systematic literature search: ["ophidiomycosis" OR "Ophidiomyces" OR "ophiodiicola" OR "ophidiicola" OR "Snake Fungal Disease" OR ("Snake" AND “Chrysosporium”) OR ("snake” AND “fungal disease") OR (“snake” AND “fungal infection”) OR (“snake” AND "keratinophilic fungus") OR (“snake” AND “mycosis”)]. To search for information regarding the presence of arthroconidia in histological sections, the following terms were also considered: “spores” (e.g., Allender et al. 2011; TWRA 2017; Guzman Vargas et al. 2020); “cylindrical” or “rectangular”; and/or “arthrospores” (e.g., Last et al. 2016; Hill et al. 2018; TWRA 2017). Duplicates were removed using Mendeley Desktop software (Version 1.19.8). Further literature was searched through secondary sources (e.g., Google Scholar) or identified by examining the references of the downloaded manuscripts.
The pre-screened documents were then further assessed to collect literature specifically dealing with cases of snakes affected by Oo and/or presenting clinical symptoms. In the selected papers, a screen was performed for each snake, in relation to clinical and/or laboratory investigations carried out to ascertain the presence of Oo and/or ophidiomycosis, including different types of PCR, DNA sequencing, and culture from cutaneous swab or tissue samples as well as histopathology and clinical signs.
All the information resulting from the screening was used to create Appendix 1, which shows all the snake species, and their organization at family level according to Zaher et al. (2019), in which the presence of the fungus has been confirmed. Data collected for each species includes case classification, geographical locations, bibliographic references, and threat level according to the International Union for Conservation of Nature (IUCN) red list.
We have based our revision on the “case classification” by Baker et al. (2019), CWHC (2017), and Davy et al. (2021), excluding cases without molecular identification of the fungus. We interpreted detection of Oo as positive only when confirmed with molecular assays (PCR or DNA sequences from tissue sample/swab or generated from Oo isolates), excluding the diagnoses based only on fungal morphology in histological sections or cultures. Snakes for which the fungal presence was diagnosed only on the basis of clinical evidence or histopathological findings (e.g., Bustos et al. (2018), reporting an alleged case from Argentina) were not included in Appendix 1.
Hence, ophidians positive for the fungus following molecular analysis only have been classified as “Oo present” (as in B.i and D.i–see section “Case classification”); cases in which gross signs were consistent with the disease along with molecular detection of Oo have been classified as “apparent Ophidiomycosis” (as in Biii.i and D.ii.i–see section “Case classification”); cases with Oo molecular positivity and histological presence of intralesional hyaline fungal hyphae (without arthroconidia) were classified as “Ophidiomycosis” (as in B.iii.ii and D.iii–see section “Case classification”); cases with Oo molecular detection and presence of fungal hyphae and arthroconidia were classified as “Ophidiomycosis and Oo shedder” (as in B.iv–see section “Case classification”).
The classification criteria used in this review are summarized in Table 1.
For ranking purposes, in circumstances in which there was not clear evidence of arthroconidia, the following assumptions were used in the following cases:
-
1.
If it was stated in the paper that histopathology was positive, we assumed that fungal hyphae were found, but not arthroconidia;
-
2.
If histopathology was positive for fungal hyphae, but it was not specified in the article whether arthroconidia were found, we assumed that arthroconidia were not found;
-
3.
If a histopathological image from a specific individual positive for molecular diagnostics included arthroconidia, we classified this specimen as “Ophidiomycosis and Oo shedder.”
Furthermore, for ranking purposes, if there was not an explicit link between molecular positivity and clinical signs or histopathologic features, we classified the specimen at a lower rank (i.e., “Oo present”).
In Appendix 1, we gave each species the highest classification rank reached in at least one specimen. The ranking followed this increasing order: (i) “Oo present”; (ii) “apparent ophidiomycosis”; (iii) “Ophidiomycosis”; (iv) “Ophidiomycosis and Oo shedder.” Note that each category comprises the parameters present at the lower level/s, except for the gross signs (see Table 1).
In order to provide an overview of the confirmed presence of the fungus around the world, a map was created to highlight the disease in free ranging and captive snakes (Fig. 5). The map was generated through the QGIS software, version 3.12.3-București by using it as a cartographic base for the USA (https://www.weather.gov/gis/USStates) and the globe (www.thematicmapping.org). The map was then further edited using Adobe Photoshop CS6.
Results
From the papers included in this review, we extracted the information to fill Appendix 1, which shows all the snake species with at least one specimen from which Oo was detected. All the states (and the single federated states for the USA) in which a positive specimen was found are also indicated for each snake species. We considered only the cases in which the presence of Oo was confirmed following molecular investigations, according to our classification criteria explained in “Materials and methods” section and summarized in Table 1, to be positive. Similarly, we considered cultures to be positive if confirmed by molecular assays (e.g., sequencing).
Oo has been detected in 62 snake species, including 24 Colubridae species, 14 Natricidae species, 6 Dipsadidae species, 7 Viperidae species, 3 Pythonidae species, 4 Boidae species, 2 Elapidae species, 1 Acrochordidae species, and 1 Homalopsidae species. Among these taxa, 20 have been categorized as “Ophidiomycosis and Oo shedder” (32.3%), 11 as “Ophidiomycosis” (17.7%), 16 as “Apparent ophidiomycosis” (25.8%), and 15 as “Oo present” (24.2%). Note that three taxa undefined at a specific level are present in Appendix 1 (Acrochordus sp., Panterophis sp., and Thamnophis sp.): Panterophis sp. and Thamnophis sp. are not considered in the count because they could be referred to as one of the species already considered, while Acrochordus sp. is included because it is certainly a univocal taxon.
The fungus has been reported in 11 states (considering the USA as a whole, where, however, the phenomenon has been registered in as many as 28 federated states) and in four of them the mycosis concerns, to date, only snakes held in captivity (Fig. 5).
For each of the 62 snake taxa involved, the categorization in the IUCN Red List of Threatened Species and the related population trends were also reported: 53 species (85.5%) are listed as LC (least concern); 3 species (4.8%) are listed as VU (vulnerable); 1 species (1.6%) is listed as EN (endangered); 4 species (6.5%) are listed as NE (not evaluated); and 1 taxon (Acrochordus sp., 1.6%) cannot be evaluated because only its generic rank is explicit. Regarding the population trend, in the 53 species listed as LC, the trend is stable for 39, decreasing for 8, and unknown for 6; in the 3 species listed as VU, the trend is decreasing for 2 and unknown for one; for the only endangered species, the trend is decreasing.
Discussion and future perspectives
According to our knowledge, the presence of Oo at a global scale has not been systematically assessed in recent times (see Lorch et al. 2016a). Furthermore, it is important to shed light on the worldwide distribution of the emergent infectious disease caused by Oo. Therefore, the primary purpose of our work was to investigate in which locations this keratinophilic fungus was detected, and, at the same time, to categorize the snake species affected by this infection.
Throughout the world, Oo-positive cases (with or without the onset of ophidiomycosis) have been recorded in only 11 states (Fig. 5, Appendix 1) in four continents (America, Asia, Europe, and Oceania). Information is completely lacking for Africa and South America and the data related to Central America, Asia, Europe, and Oceania are limited. Therefore, further investigations will be essential to understand where Oo is a matter of concern. From our review, Oo has been detected in 62 species of ophidians all over the world, a high number that should raise awareness among scientists in this field. The host spectrum and diversity of affected snake species seems to be increasing, consistent with the hypothesis of spatial ubiquity of Oo (Burbrink et al. 2017). This may also be due to the increasing number of Ophidiomycosis surveillance projects, at least in the Nearctic ecozone, during the years after the threat emergence (Davy et al. 2021).
Classification of the cases has the primary aim of helping to summarize diagnostic results and standardize different diagnostic pictures in order to make them comparable. Nonetheless, beyond harmonization, the significance of each category in terms of epidemiology and disease ecology might be overlooked. Hereinafter we discuss the relevance of the diagnostic criteria used for each snake species in this review. Note that each category comprises the parameters of the lower levels, except for gross signs (see Table 1):
-
i.
“Oo present” is the category in which fungal DNA is detected, indicating the presence of the fungus. However, Oo elements or fungal DNA might be a consequence of environmental contamination. Furthermore, the presence of Oo in an individual with intact tegument could imply good host resistance, hence, an ability to block pathogen entry or to restrict pathogen replication. However, it cannot be excluded that such individuals are more prone to visceral Oo infections without showing external signs when chronically exposed to the pathogen found on the skin surface. In the same way, it cannot be excluded that the presence of the fungus is related to pre-clinical infection, colonization, or residual fungus subsequent to the healing process.
-
ii.
“Apparent ophidiomycosis” is the category comprising the presence of the fungus and a potential causality relationship with gross, usually cutaneous, lesion/s consistent with Oo mycosis.
-
iii.
“Ophidiomycosis” is the category in which the fungal DNA has been detected along with hyphae consistent with Oo seen on histology. It is unknown if a certain species or individual can be infected, permitting Ophidiomyces to form branching hyphae and/or fungaemia/dissemination, without the formation of the infective propagule (i.e., arthroconidium). In this case, the infected snake would act as a “dead end host” that does not allow the fungus to be spread in the environment and thus transmitted to other animals. Additionally, non-cutaneous forms of ophidiomycosis, such as the visceral/pulmonary form that does not display skin lesions, and consequently infective propagules on the air-skin interface (e.g., Steeil et al. 2018a), might not allow the detection of arthroconidia even after tegument biopsy, misleading the diagnosis if classified according to Baker et al. (2019). Moreover, in “Oo positive” histological sections, while hyphae are always reported and arthroconidia presence is common, arthroconidial tufts only occur sometimes at the surface of the lesion (Paré and Sigler 2016). Therefore, the arthroconidia are not always present (e.g., Sigler et al. 2013) and their aggregates (arthroconidial tufts) considered “practically pathognomonic” (Paré and Sigler 2016) are less frequent than the isolated spores.
-
iv.
“Ophidiomycosis and Oo shedder”: the species and the individuals in this category are able to shed Oo into the environment, confirmed by the histological detection of the propagule. They should, therefore, be able to transmit the infection and infect other animals. However, it is still unknown what role they have in epidemic outbreaks, and if, having a high infectivity and resistance at the same time (implicating a greater risk of transmission), they should perhaps be categorized as “spreaders” or “super spreaders”.
The present classification of cases in which Oo has been at least detected aims to provide a standardization tool for future studies and to aid conclusive diagnosis in cases of potential ophidiomycosis. However, it has the following limitations:
-
The genus Paranannizziopsis includes four species, all former members of the CANV complex and closely related to Oo, that are able to infect aquatic snakes from the species Erpeton tentaculatum and Acrochordus sp. (Paré and Sigler 2016). In particular, P. crustacea produce hyphae and arthroconidia that overlap with features of Oo (Sigler et al. 2013). Therefore, mixed infections could mislead the diagnosis if, for example, results are obtained with Oo-specific PCR primers and histochemistry.
-
Identification of fungal elements in tissues may be challenging during visceral or deep dermal infections or in cases with significant tissue necrosis.
The ideal tool to confirm the identity of Oo in the tissues is in situ DNA hybridization, a method not yet available for Oo (Paré and Sigler 2016).
From a clinical-pathological point of view, another limitation to this classification tool is a lack of comprehensive information about the immune response elicited by Oo infection, which can only be assessable via a complete anatomo/histopathological examination and selected ancillary tests. This approach might help to distinguish between saprophytism, colonization, infection, and disease. It is still unknown how the ability to restrict Oo replication, i.e., resistance (Romani 2011), and the ability to limit the detrimental impact of Oo infection without affecting pathogen burden per se, i.e., protective tolerance (Romani 2011), is balanced by the immune system of different snake hosts in different conditions. The resulting (dys)equilibrium between pro-inflammatory and anti-inflammatory responses influences the ability of an individual to spread and transmit the infection (infectivity). Moreover, the balance of host responses can determine transition from saprophytism to infection, especially in mycosis (Romani and Puccetti 2006; Romani 2011).
We still do not know the mechanism by which Oo causes mortality in wild snakes, although it is very likely that development of infection is multifactorial (McKenzie et al. 2020a, b). Further studies are needed to understand whether Oo is a primary pathogen or if it has a secondary role and whether it is an opportunistic fungus causing pathology by evading or subverting host inflammation. Unfortunately, there are practical issues when studying antifungal mediators, such as leukocytes and cytokines (Romani and Puccetti 2006), in ectothermic vertebrates, including reptiles (Zimmerman et al. 2010). An example is the poor cross-reactivity between antibodies against cytokines of mammalian and of ectothermic origins (Scapigliati et al. 2006).
From the papers analyzed for this review, it would have been useful to have laboratory data and information on clinical signs (see Baker et al. 2019) for each snake observed. The lack of, or discrepancies in, these data did not allow us to perform a meta-analysis in this direction. Therefore, the authors suggest that future reports or studies on ophidiomycosis should use, when possible, a more standardized and detailed method of recording laboratory and clinical information at the level of each individual snake.
Hence, we propose a list of information that should be recorded and published for each Oo-positive snake:
-
individual ID code;
-
species;
-
date and time of discovery;
-
wild or captive condition;
-
location coordinates;
-
approximate age class;
-
snout-vent length;
-
specimen status (live, dead, moulted skin);
-
clinical signs (Y/N and specify);
-
gross lesions (Y/N and specify);
-
skin swab/sample PCR outcome (Y/N/not performed);
-
culture outcome (Y/N/not performed);
-
culture molecular confirmation (Y/N/not performed);
-
histology (Y/N/not performed);
-
histology outcome (e.g., presence of intralesional hyaline fungal hyphae and arthroconidia).
Scoring systems should be used in the future to harmonize results, not only within a single study but also between different studies. An obvious obstacle to this objective is the multi-host nature and the broad range of Oo; for this reason, data harmonization should always take into account the different ophidian species and should be designed and driven on a species-by-species basis (Baker et al. 2019).
Furthermore, for populations of a given species in which it is also possible to collect a substantial number of individuals in good health for comparison, it is important to calculate the BCI. This allows researchers to extrapolate information on energy reserves and adipose tissue, which is useful to correlate the amount of adipose reserve with the severity of the infection (see McCoy et al. 2017; Lind et al. 2018b). Low levels of fat reserves, due to lower acquisition or higher consumption of resources, may establish the vicious cycle potentially triggered by this disease (Lind et al. 2018c).
To better understand the dynamics and the extent of spread of Oo around the world, more field surveys are recommended, especially in those states where the presence of the fungus has not yet been sufficiently investigated. Zoologists and naturalists involved in environmental and faunal monitoring should be trained to recognize and eventually sample a suspected case of Oo infection. Well-planned quantitative and field-based approaches specific for species and location (e.g., McKenzie et al. 2021) are encouraged.
To date, a close relationship between the occurrence of ophidiomycosis and decrease in certain snake populations has not yet been demonstrated. Nevertheless, different species of snakes have an ecology that does not make their detectability easy. These species are difficult to study and evaluate at a population level, particularly in the short term (Turner 1978; Ward et al. 2017). Therefore, the effects of ophidiomycosis on population trends may not always be easy to assess.
Given the ecology and the potential elusivity of the pathogen itself, Oo should be considered potentially dangerous for most of the populations in which it is present. Its occurrence within populations already in decline or with a reduced range should be even more alarming. In these situations, the individuals are few and they may already be exposed to other stressors, such as genetic isolation, inbreeding, and exposure to xenobiotics and stochastic events (De Castro and Bolker 2005; Clark et al. 2011; EFSA 2018), that may increase their susceptibility to ophidiomycosis (e.g., through an inability to mount an adequate immune response). Moreover, in a small population with a restricted distribution, disease may induce extinction since recovery via recruitment, recolonization, and immigration is less likely (De Castro and Bolker 2005; Bielby et al. 2008). Therefore, particular attention should be paid to taxa with an extremely reduced and disjointed area (e.g., Vipera ursinii) or endangered island endemisms (e.g., Macrovipera schweizeri).
The studies in which Oo has been evaluated as not impacting the survival of the species are few (i.e., Dillon 2020; Mckenzie et al. 2021) and concern only a few taxa. Therefore, further investigation is needed to assess the impact of this fungus on snake fitness, especially considering that it could vary between different taxa and environmental contexts, and that the development of Oo infection is likely multifactorial in wild snakes (Lorch et al. 2016a; Lind et al. 2018c; McKenzie et al. 2020a).
The best period for field surveys will depend on the ecology of the investigated species. For snakes living in temperate belts, which are characterized by marked seasonality and where ophidians overwinter, the field work should be mostly realized during the peri-brumation period after emergence and before returning to overwintering. During this period, infected ophidians are still basking while uninfected snakes have retreated in brumation refugia (Tetzlaff et al. 2017; Dillon 2020). If screening is conducted during unfavorable time periods, when ophidians show low Oo prevalence and/or can clear the cutaneous form of Ophidiomycosis and/or the fungus is absent or not detectable from skin swabs, the site might be incorrectly declared as “ophidiomycosis-free” (Dillon 2020).
Furthermore, to improve knowledge of the spread of the fungus, it is also important to carry out retrospective surveys on snakes kept in museum collections (Allender et al. 2016; Lorch et al. 2021), both through preliminary macroscopic observation of clinical signs and by laboratory analysis.
Within the Oo species, two out of three identified clades have been found in at least two different world macro areas (Franklinos et al. 2017; Sun et al. 2021), but it is not yet clear why snakes from different locations were affected by fungal strains of the same clade. This might be explained by the translocation of an affected ophidian or the pathogen itself via vectors or fomites. Therefore, to better understand the distribution, evolution, and origins of Oo, it is advisable to continue with the molecular phylogenetic analysis of new isolates, especially from those areas where data are still lacking. This may be carried out following phylogenetics methods such as those performed by Franklinos et al. (2017) and Sun et al. (2021), and the methods used by Ladner et al. (2022).
To date, it is not clear whether climate change has exacerbated the manifestation of reptile diseases caused by Oo or other closely related Onygenaceae and Nannizziopsiaceae, or whether the increased prevalence is due to underdiagnosis or misdiagnosis in the past (Stchigel et al. 2013). Therefore, future studies are needed to assess on the disease ecology of these fungi.
Although wild snakes are generally protected by laws that prohibit their manipulation and capture in the absence of specific authorizations (Geniez 2018; Di Nicola et al. 2021), they are frequently the object of attention by enthusiasts who do “herping” activities for unauthorized recreational purposes (e.g., wildlife photography following manipulation to pose the subjects and collection for terrarium purposes, as demonstrated by the contents published daily on forums and sector groups in social networks). Considering that Oo might be spread by animate and inanimate vectors, it is recommended that the authorities monitor compliance in terms of unauthorized manipulation or collection of wild snakes, as well as the release of captive snakes in the wild. This is needed to limit transmission of the pathogen in new habitats and snake populations (see Ladner et al. 2022), especially those small in size and/or occurring in a restricted area. At the same time, captive snake holders and recovery centers should always consider disease risk prevention and assessment and should perform a quarantine of at least 90 days and a microbiological screening for Oo when a new individual enters the collection (Rivera 2019; Rossi 2019). Furthermore, the use of effective disinfectants against Oo to minimize cross-contamination, as indicated by Rzadkowska and colleagues (2016), is highly recommended.
Histopathological diagnosis of Ophidiomycosis may be challenging. The HE stain routinely used in histopathology might highlight fungal hyphae and arthroconidia, especially, and sometimes only (Dolinski et al. 2014; Haynes et al. 2021), at the air-tissue interface of cutaneous or internal organs. Histochemical stains, such as silver stains (e.g., Gomori’s or Grocott’s methenamine silver) or periodic acid–Schiff are used to better characterize the morphology of fungal elements (Fig. 2–Baker et al. 2019). The latter histochemical stains appear to be more sensitive and suitable in detecting Ophidiomyces (e.g., in necrotic cores of fungal granulomas) (Dolinski et al. 2014; Last et al. 2016; Ohkura et al. 2016; Steeil et al. 2018a). These stains should be always used in suspected cases of fungal infection when routine staining does not show fungal elements (especially arthroconidia), particularly in granulomas. Moreover, a silver staining counterstained with HE should be able to leverage the qualities of both dyes (see Vissiennon et al. 1999).
Complete physical examination of snakes by a field veterinarian, with the intent to raise suspicion, particularly of non-cutaneous or subclinical forms of infections (e.g., Steeil et al. 2018a), should be an essential part of the screening. Studies targeting the systemic dissemination of the fungus are needed to evaluate associations between cutaneous and visceral infection. Similarly, hematogenous fungal spread could be confirmed with ad hoc investigations using blood culture (Pohly 2020), a method already used for other purposes in serpentes (Waugh et al. 2017). Furthermore, complete necropsy and histopathology of all organs should be performed to detect Oo in the tissues.
The elusivity of the disease in newborns, which show subtle or absent clinical signs (Britton et al. 2019), should raise concern because it can bring “silent deaths.” Investigation on this fundamental age class should be done, shedding light on the transmission route and on the impact on species’ fitness and demography (Britton et al. 2019; Stengle et al. 2019; McKenzie et al. 2020b).
Based on the potential role of hibernacula to act as pathogen reservoirs (Campbell et al. 2021), an experimental study with uninfected snakes exposed to different types of substrates with the absence/presence of infected individuals should be performed to elucidate the mode of transmission of Oo within a common hibernaculum. Moreover, studies should assess whether the capability of hibernacula to preserve Oo propagules (conidia) is caused by soil properties and/or by fungal shedding from brumating snakes (Campbell et al. 2021).
Beyond various suggestions that the reader can find within the text above, we would like to propose a concise structure for Oo diagnostics with the aim of assisting preliminary surveys, particularly in countries with a lack of data on the fungus, and harmonizing future wildlife research on Oo. This is also summarized in Table 2.
The record of clinical signs in the field, as well as gathering of observations from other sources (e.g., local citizens, social media, and public databases), is a prerequisite to focus the effort in a circumscribed area. Likewise, the organization of materials and coordination of an interdisciplinary team (Allain and Duffus 2019) is important before starting a study. International cooperation can be very fruitful.
Molecular detection of Oo has priority in surveillance. Dry swabs are advised for molecular screening of the fungus. The operator should use moderate pressure to pass the cotton tip of a sterile wooden applicator longitudinally over the head region, dorsal scales, and ventral scales, 10 times, trying to cover the entire skin surface. In case of lesions consistent with Oo mycosis, each lesion should be swabbed 10 times, covering the entire affected area. The use of a single swab is strongly discouraged; in most cases, triplicate swabbing is time feasible and has the best cost–benefit ratio in term of outcomes. This approach will result in fewer false negatives and improved reproducibility because, after DNA extraction, a PCR run in duplicate or triplicate will yield six to nine results, respectively, per individual. Tissues and exuviae can be used for molecular detection, as well as scale clips (McKenzie et al. 2019). We suggest the use of specific Oo primers targeting the internal transcribed spacer region (Bohuski et al. 2015; Allender et al. 2015b), that, so far, seem the most sensitive and employed in qPCR.
The second diagnostic priority is histopathology. While a complete necropsy is ideal to apply the standardized scoring systems, it is not always possible. In our experience, scale clipping as a method of tissue sampling and subsequent processing for histology is feasible to assess the presence and the morphology of fungal hyphae and arthrospores (unpubl. data–see Fig. 2). It is minimally invasive and can also be used for molecular purposes. Nevertheless, scale clipping compromises the integrity of the skin and may facilitate the invasion of Oo into deeper tissues (Lorch et al. 2015; McKenzie et al. 2019). For this reason, this kind of tissue sampling as well as punch biopsies is not recommended in free-ranging ophidians that are released prior to an imminent hibernation. PAS or silver staining should be used to highlight fungi in tissue sections.
While culture is almost indispensable to molecularly characterize the fungus, it is not a principal goal for a preliminary screening. However, storage of tissues or moults at − 20 °C is advisable for prospective analysis. The use of swabs should be avoided for fungal culture (Davy et al. 2021).
For further clarifications see the “Standardized approaches” section and referenced literature therein.
In conclusion, this review summarizes reported Oo-positive cases and current distribution data. We also provide guidelines on a how to standardize methods for future studies on the prevalence, distribution, and pathogenesis of ophidiomycosis in ophidiofauna. Standardized methods should become a cornerstone for diagnosis and should be prospectively pursued in a global context. Further studies on the origin and epidemiology/disease ecology of Oo through the different taxa and biogeographic realms are needed in order to safeguard wild snakes from this silent, emerging, and still incompletely characterized, infectious disease.
References
Agugliaro J, Lind CM, Lorch JM, Farrell TM (2020) An emerging fungal pathogen is associated with increased resting metabolic rate and total evaporative water loss rate in a winter-active snake. Func Ecol 34:486–496. https://doi.org/10.1111/1365-2435.13487
Allain SJ, Duffus AL (2019) Emerging infectious disease threats to European herpetofauna. Herpetol J 29:189–206. https://doi.org/10.33256/hj29.4.189206
Allender MC, Dreslik M, Wylie S, Phillips C, Wylie DB, Maddox C, Kinsel MJ (2011) Chrysosporium sp. infection in eastern massasauga rattlesnakes. Emerg Infectious Dis 17:2383. https://doi.org/10.3201/eid1712.110240
Allender MC, Baker S, Wylie D, Loper D, Dreslik MJ, Phillips CA, Maddox C, Driskell EA (2015a) Development of snake fungal disease after experimental challenge with Ophidiomyces ophiodiicola in cottonmouths (Agkistrodon piscivorous). PLoS ONE 10:e0140193. https://doi.org/10.1371/journal.pone.0140193
Allender MC, Bunick D, Dzhaman E, Burrus L, Maddox C (2015b) Development and use of a real-time polymerase chain reaction assay for the detection of Ophidiomyces ophiodiicola in snakes. J Vet Diagn Invest 27:217–220. https://doi.org/10.1177/1040638715573983
Allender MC, Raudabaugh DB, Gleason FH, Miller AN (2015c) The natural history, ecology, and epidemiology of Ophidiomyces ophiodiicola and its potential impact on free-ranging snake populations. Fungal Ecol 17:187–196. https://doi.org/10.1016/j.funeco.2015.05.003
Allender MC, Phillips CA, Baker SJ, Wylie DB, Narotsky A, Dreslik MJ (2016) Hematology in an eastern massasauga (Sistrurus catenatus) population and the emergence of Ophidiomyces in Illinois, USA. J Wildl Dis 52:258–269. https://doi.org/10.7589/2015-02-049
Allender MC, Baker S, Britton M, Kent AD (2018) Snake fungal disease alters skin bacterial and fungal diversity in an endangered rattlesnake. Sci Rep-Uk 8:1–9. https://doi.org/10.1038/s41598-018-30709-x
Allender MC, Ravesi MJ, Haynes E, Ospina E, Petersen C, Phillips CA, Lovich R (2020) Ophidiomycosis, an emerging fungal disease of snakes: targeted surveillance on military lands and detection in the western US and Puerto Rico. PLoS ONE 15:e0240415. https://doi.org/10.1371/journal.pone.0240415
Baker SJ, Haynes E, Gramhofer M, Stanford K, Bailey S, Christman M, Allender MC (2019) Case definition and diagnostic testing for snake fungal disease. Herpetol Rev 50:279–285
Baker SJ, Niemiller ML, Stites AJ, Ash KT, Davis MA, Dreslik MJ, Phillips CA (2020) Evaluation of environmental DNA to detect Sistrurus catenatus and Ophidiomyces ophiodiicola in crayfish burrows. Conserv Genet Resour 12:13–15. https://doi.org/10.1007/s12686-018-1053-9
Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N (2008) Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun 76:907–915. https://doi.org/10.1128/IAI.01432-07
Bicknese E (2009) Itraconazole treated CANV (Chrysosporium anamorph of Nannizziopsis vriesii) in green anacondas (Eunectes murinus murinus). Proc ARAV:157–158
Bielby J, Cooper N, Cunningham A, Garner TWJ, Purvis AJCL (2008) Predicting susceptibility to future declines in the world’s frogs. Conserv 1:82–90. https://doi.org/10.1111/j.1755-263X.2008.00015.x
Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbole KPC, Harris RN (2013) Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecol Lett 16:807–820. https://doi.org/10.1111/ele.12099
Bletz MC, Kelly M, Sabino-Pinto J, Bales E, Van Praet S, Bert W, Boyen F, Vences M, Steinfartz S, Pasmans F, Martel A (2018) Disruption of skin microbiota contributes to salamander disease. Proc Biol Sci 285:20180758. https://doi.org/10.1098/rspb.2018.0758
Böhm M, Collen B, Baillie JE, Bowles P, Chanson J, Cox N, Mateo JA et al (2013) The conservation status of the world’s reptiles. Biol Conserv 157:372–385. https://doi.org/10.1016/j.biocon.2012.07.015
Bohuski E, Lorch JM, Griffin KM, Blehert DS (2015) TaqMan real-time polymerase chain reaction for detection of Ophidiomyces ophiodiicola, the fungus associated with snake fungal disease. BMC Vet Res 11(1):1–10. https://doi.org/10.1186/s12917-015-0407-8
Britton M, Allender MC, Hsiao SH, Baker SJ (2019) Postnatal mortality in neonate rattlesnakes associated with Ophidiomyces ophiodiicola. J Zoo Wildlife Med 50:672–677. https://doi.org/10.1638/2018-0198
Burbrink FT, Lorch JM, Lips KR (2017) Host susceptibility to snake fungal disease is highly dispersed across phylogenetic and functional trait space. Sci Adv 3(12):e1701387. https://doi.org/10.1126/sciadv.1701387
Burns G, Ramos A, Muchlinski A (1996) Fever response in North American snakes. J Herpetol. https://doi.org/10.2307/1565503
Bustos ML, Nicolas Sanchez M, Peichoto ME, Teibler GP (2018) First report of fungal disease in a South American snake. RIVEP 29:1036–1042. https://doi.org/10.15381/rivep.v29i3.15072
Campbell LJ, Burger J, Zappalorti RT, Bunnell JF, Winzeler ME, Taylor DR, Lorch JM (2021) Soil reservoir dynamics of Ophidiomyces ophidiicola, the causative agent of snake fungal disease. J Fungus 7(6):461
Chandler HC, Allender MC, Stegenga BS, Haynes E, Ospina E, Stevenson DJ (2019) Ophidiomycosis prevalence in Georgia’s Eastern indigo snake (Drymarchon couperi) populations. PLoS ONE 14:e0218351. https://doi.org/10.1371/journal.pone.0218351
Clark RW, Marchand MN, Clifford BJ, Stechert R, Stephens S (2011) Decline of an isolated timber rattlesnake (Crotalus horridus) population: interactions between climate change, disease, and loss of genetic diversity. Biol Conserv 144:886–891. https://doi.org/10.1016/j.biocon.2010.12.001
CWHC (2017) Snake fungal disease in Canada rapid threat assessment. Canadian Wildlife Health Cooperative. http://www.cwhc-rcsf.ca/docs/technical_reports/CWHC_Snake_Fungal_Disease_Threat_Assessment.pdf. Accessed 26 April 2021
Davy CM, Shirose L, Campbell D, Dillon R, McKenzie C, Nemeth N, Braithwaite T, Cai H, Degazio T, Dobbie T, Egan S, Fotherby H, Litzgus11 JD, Manorome P, Marks S, Paterson JE, Sigler L, Slavic D, Slavik E, Urquhart J, Jardine C (2021) Revisiting ophidiomycosis (snake fungal disease) after a decade of targeted research. Front Vet Sci 8:448. https://doi.org/10.3389/fvets.2021.665805
De Castro F, Bolker B (2005) Mechanisms of Disease-Induced Extinction Ecol 8:117–126. https://doi.org/10.1111/j.1461-0248.2004.00693.x
Di Nicola MR, Cavigioli L, Luiselli L, Andreone F (2021) Anfibi & Rettili d'Italia. Edizioni Belvedere, Latina
Dibadj B, Haynes E, Vivirito K, Wright A, Stanford K, Allender MC (2021) Investigating agreement between snake sheds and skin swabs in detection of Ophidiomyces ophidiicola. J Herpetol Med Surg. https://doi.org/10.1111/JHMS-S-20-00007
Dillon (2020) Disease ecology of ophidiomycosis in free-ranging snakes. Master’s thesis, Trent University, Peteroborugh, Ontario
Dolinski AC, Allender MC, Hsiao V, Maddox CW (2014) Systemic Ophidiomyces ophiodiicola infection in a free-ranging plains garter snake (Thamnophis radix). J Herpetol Med Surg 24:7–10. https://doi.org/10.5818/1529-9651-24.1.7
EFSA (2018) Scientific Opinion on the state of the science on pesticide risk assessment for amphibians and reptiles. EFSA J 16:5125. https://doi.org/10.2903/j.efsa.2018.5125
Ficetola GF, Miaud C, Pompanon F, Taberlet P (2008) Species detection using environmental DNA from water samples. Biol Lett 4:423–425. https://doi.org/10.1098/rsbl.2008.0118
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194. https://doi.org/10.1038/nature10947
Franklinos LH, Lorch JM, Bohuski E, Fernandez JRR, Wright ON, Fitzpatrick L, Petrovan S, Durrant C, Linton C, Baláž V, Cunningham AA, Lawson B (2017) Emerging fungal pathogen Ophidiomyces ophiodiicola in wild European snakes. Sci Rep-Uk 7:1–7. https://doi.org/10.1038/s41598-017-03352-1
Fredrickson K (2019) Snake fungal disease in Lake Erie water snakes and its effect on attitude and behavior during treatment. Celebration of Learning
Fuchs LD, Tupper TA, Aguilar R, Lorentz EJ, Bozarth CA, Fernandez DJ, Lawlor DM (2020) Detection of Ophidiomyces ophiodiicola at two mid-Atlantic natural areas in Anne Arundel County, Maryland and Fairfax County, Virginia, USA. Amphib Reptile Conse 14:22–28
GBIF Secretariat (2019) Ophidiomyces ophiodiicola (Guarro, Deanna A.Sutton, Wickes & Rajeev) Sigler L, Hambleton S, Paré JA (2013). GBIF Backbone Taxonomy. Checklist dataset https://doi.org/10.15468/39omei . https://www.gbif.org/species/8200173 . Accessed 20 July 2021
Geniez P (2018) Snakes of Europe. Princeton, North Africa and the Middle East. A photographic guide University Press, New Jersey
Glorioso BM, Waddle JH, Green DE, Lorch JM (2016) First documented case of snake fungal disease in a free-ranging wild snake in Louisiana. Southeast Nat. https://doi.org/10.1656/058.015.0111
Glorioso B, Bartoszek IA, Lorch JM (2020) Low-level detection of SFD-causing Ophidiomyces on Burmese pythons in southwest Florida, with confirmation of the pathogen on co-occurring native snakes
Grisnik M, Leys JE, Bryan D, Hardman RH, Miller DL, Cobb VA, Walker DM (2018) Host and geographic range of snake fungal disease in Tennessee, USA. Herpetol Rev 49:682–690
Guthrie (2016) Update on snake fungal disease in Eastern Virginia. Catesbeiana 36
Guthrie AL, Knowles S, Ballmann AE, Lorch JM (2016) Detection of snake fungal disease due to Ophidiomyces ophiodiicola in Virginia, USA. J Wildlife Dis 52:143–149. https://doi.org/10.7589/2015-04-093.1
Guzman-Vargas V, Alexander JL, Fenton HMA, Niedringhaus KD, Allender MC, Shender LA (2020) Ophidiomycosis in Florida’s wild snakes: state records and treatment of an infected Nerodia taxispilota. Herpetol Rev 51:47–51
Harris RN, James TY, Lauer A, Simon MA, Patel A (2006) Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth 3:53–56. https://doi.org/10.1007/s10393-005-0009-1
Haynes E, Chandler HC, Stegenga BS, Adamovicz L, Ospina E, Zerpa-Catanho D, Allender MC (2020) Ophidiomycosis surveillance of snakes in Georgia, USA reveals new host species and taxonomic associations with disease. Sci Rep-Uk 10:1–15. https://doi.org/10.1038/s41598-020-67800-1
Haynes E, Pohly A, Clifford DL, Patterson LC, Manning S, Wack RF, Allender MC (2021) First report of Ophidiomycosis in a free-ranging California Kingsnake (Lampropeltis californiae) in California, USA. J Wildl Dis 57:246–249. https://doi.org/10.7589/JWD-D-20-00006
Hileman ET, Allender MC, Bradke DR, Faust LJ, Moore JA, Ravesi MJ, Tetzlaff SJ (2018) Estimation of Ophidiomyces prevalence to evaluate snake fungal disease risk. J Wildlife Manage 82:173–181. https://doi.org/10.1002/jwmg.21345
Hill AJ, Leys JE, Bryan D, Erdman FM, Malone KS, Russell GN, Walker DM (2018) Common cutaneous bacteria isolated from snakes inhibit growth of Ophidiomyces ophiodiicola. EcoHealth 15:109–120. https://doi.org/10.1007/s10393-017-1289-y
Jacobson ER (2007) Overview of reptile biology, anatomy, and histology. In: Jacobson ER (ed) Infectious diseases and pathology of reptiles: Color Atlas and Text. CRC Press, Florida, pp 131–166
Jani AJ, Briggs CJ (2014) The pathogen Batrachochytrium dendrobatidis disturbs the frog skin microbiome during a natural epidemic and experimental infection. PNAS 111:E5049–E5058. https://doi.org/10.1073/pnas.1412752111
Januszkiewicz E, LaDuke TC, Chinnici N (2020) Distribution and severity of snake fungal disease caused by Ophidiomyces ophiodiicola among timber rattlesnakes (Crotalus horridus) in Pennsylvania. Toxicon 182:S31. https://doi.org/10.1016/j.toxicon.2020.04.077
Kirk P (2020) Ophidiomyces ophidiicola. Index Fungorum. http://www.indexfungorum.org/names/NamesRecord.asp?RecordID=637774. Accessed 20 July 2021
Labocha MK, Schutz H, Hayes JP (2014) Which body condition index is best? Oikos 123:111–119. https://doi.org/10.1111/j.1600-0706.2013.00755.x
Ladner JT, Palmer JM, Ettinger CL, Stajich JE, Farrell TM, Glorioso BM, Lawson B, Price SJ, Stengle AG, Grear DA, Lorch JM (2022) The population genetics of the causative agent of snake fungal disease indicate recent introductions to the USA. PLoS Biol 20:e3001676. https://doi.org/10.1371/journal.pbio.3001676
Langwig KE, Frick WF, Hoyt JR, Parise KL, Drees KP, Kunz TH, Kilpatrick AM (2016) Drivers of variation in species impacts for a multi-host fungal disease of bats. Philos Trans R Soc Lond B Biol Sci 371:20150456. https://doi.org/10.1098/rstb.2015.0456
Last LA, Fenton H, Gonyor-McGuire J, Moore M, Yabsley MJ (2016) Snake fungal disease caused by Ophidiomyces ophiodiicola in a free-ranging mud snake (Farancia abacura). J Vet Diagn Invest 28:709–713. https://doi.org/10.1177/1040638716663250
Lauer A, Simon MA, Banning JL, Andre E, Duncan K, Harris RN (2007) Common cutaneous bacteria from the eastern redbacked salamander can inhibit pathogenic fungi. Copeia 3:630–640. https://doi.org/10.1371/journal.pone.0010957
Licitra D, Quinn DP, Reeder JE, Gavitt T, Dickson J, Hess B, Szczepanek SM (2019) Snake fungal disease in colubridae snakes in Connecticut, USA in 2015 and 2017. J Wildlife Dis 55:658–662. https://doi.org/10.7589/2018-04-100
Lind CM, Lorch JM, Moore IT, Vernasco BJ, Farrell TM (2018a) Seasonal sex steroids indicate reproductive costs associated with snake fungal disease. J Zool 307:104–110. https://doi.org/10.1111/jzo.12628
Lind CM, McCoy CM, Farrell TM (2018b) Tracking outcomes of snake fungal disease in free-ranging pygmy rattlesnakes (Sistrurus miliarius). J Wildlife Dis 54:352–356. https://doi.org/10.7589/2017-05-109
Lind CM, Moore IT, Akçay Ç, Vernasco BJ, Lorch JM, Farrell TM (2018c) Patterns of circulating corticosterone in a population of rattlesnakes afflicted with snake fungal disease: stress hormones as a potential mediator of seasonal cycles in disease severity and outcomes. Physiol Biochem Zool 91:765–775. https://doi.org/10.1086/695747
Lind CM, Clark A, Smiley-Walters SA, Taylor DR, Isidoro-Ayza M, Lorch JM, Farrell TM (2019) Interactive effects of food supplementation and snake fungal disease on pregnant pygmy rattlesnakes and their offspring. J Herpetol 53:282–288. https://doi.org/10.1670/18-147
Lizarraga A (2020) Prevalence of snake fungal disease caused by Ophidiomyces ophiodiicola in east Texas. Master’s thesis, University of Texas at Tyler, Tyler, Texas
Long RB, Love D, Seeley KE, Patel S, Allender MC, Garner MM, Ramer J (2019) Host factors and testing modality agreement associated with Ophidiomyces infection in a free-ranging snake population in southeast Ohio, USA. J Zoo Wildlife Med 50:405–413. https://doi.org/10.1638/2018-0143
Lorch JM, Knowles S, Lankton JS, Michell K, Edwards JL, Kapfer JM, Blehert DS (2016a) Snake fungal disease: an emerging threat to wild snakes. Philos T Roy Soc B 371:20150457. https://doi.org/10.1098/rstb.2015.0457
Lorch JM, Knowles S, Lankton JS, Michell K, Edwards JL, Kapfer JM, Blehert DS (2016b) Snake dermatitis data. USGS Data Release. https://doi.org/10.5066/F7Z31WRB
Lorch JM, Lankton J, Werner K, Falendysz EA, McCurley K, Blehert DS (2015) Experimental infection of snakes with Ophidiomyces ophiodiicola causes pathological changes that typify snake fungal disease. MBio 6(6). https://doi.org/10.1128/mbio.01534-15
Lorch JM, Price SJ, Lankton JS, Drayer AN (2021) Confirmed cases of Ophidiomycosis in museum specimens from as early as 1945. United States Emerg Infect Dis. https://doi.org/10.3201/eid2707.204864
Meier G, Notomista T, Marini D, Ferri V (2018) First case of snake fungal disease affecting a free-ranging Natrix natrix (Linnaeus, 1758) in Ticino Canton, Switzerland. Herpetol Notes 11:885–891
McBride MP, Wojick KB, Georoff TA, Kimbro J, Garner MM, Wang X, Wellehan JF (2015) Ophidiomyces ophiodiicola dermatitis in eight free-ranging timber rattlesnakes (Crotalus horridus) from Massachusetts. J Zoo Wildlife Med 46:86–94. https://doi.org/10.1638/2012-0248R2.1
McCoy CM, Lind CM, Farrell TM (2017) Environmental and physiological correlates of the severity of clinical signs of snake fungal disease in a population of pigmy rattlesnakes. Sistrurus Miliarius Conserv Physiol. https://doi.org/10.1093/conphys/cow077
McKenzie JM, Price SJ, Fleckenstein JL, Drayer AN, Connette GM, Bohuski E, Lorch JM (2019) Field diagnostics and seasonality of Ophidiomyces ophiodiicola in wild snake populations. EcoHealth 16:141–150. https://doi.org/10.1007/s10393-018-1384-8
McKenzie CM, Oesterle PT, Stevens B, Shirose L, Lillie BN, Davy CM, Nemeth NM (2020a) Pathology associated with ophidiomycosis in wild snakes in Ontario. Canada Can Vet J 61:957
McKenzie CM, Oesterle PT, Stevens B, Shirose L, Mastromonaco GF, Lillie BN, Davy CM, Jardine CM, Nemeth NM (2020b) Ophidiomycosis in red cornsnakes (Pantherophis guttatus): potential roles of brumation and temperature on pathogenesis and transmission. Vet Pathol 57:825–837. https://doi.org/10.1177/0300985820953423
McKenzie JM, Price SJ, Connette GM, Bonner SJ, Lorch JM (2021) Effects of snake fungal disease on short-term survival, behavior, and movement in free-ranging snakes. Ecol Appl 31:e02251. https://doi.org/10.1002/eap.2251
McLelland DJ, Johnson L, Reuter R (2010) Fatal cutaneous mycosis in a broad-headed snake (Hoplocephalus bungaroides) caused by the Chrysosporium anamorph of Nannizziopsis vriesii. Proc Wildl Dis Assoc 55
Nichols DK, Weyant RS, Lamirande EW, Sigler L, Mason RT (1999) Fatal mycotic dermatitis in captive brown tree snakes (Boiga irregularis). J Zoo Wildlife Med 111–118
Nilsson RH, Larsson KH, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Glöckner FO, Tedersoo L, Saar I, Kõljalg U, Abarenkov K (2019) The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res 47:D259-D264. https://doi.org/10.1093/nar/gky1022 . https://unite.ut.ee/bl_forw_sh.php?sh_name=SH1557774.08FU#fndtn-panel1. Accessed 20 July 2021
O'Hanlon SJ, Rieux A, Farrer RA, Rosa GM, Waldman B, Bataille A, Fisher MC et al (2018) Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360:621e627. https://doi.org/10.1126/science.aar1965
Ohkura M, Fitak RR, Wisecaver JH, DeBlasio D, Niazi F, Egholm M, Rounsley SD, Kodira CD, Orbach MJ (2017) Genome sequence of Ophidiomyces ophiodiicola, an emerging fungal pathogen of snakes. Genome Announc 5:e00677–17. https://doi.org/10.1128/genomeA.00677-17
Ohkura M, Worley JJ, Hughes-Hallett JE, Fisher JS, Love BC, Arnold AE, Orbach MJ (2016) Ophidiomyces ophiodiicola on a captive black racer (Coluber constrictor) and a garter snake (Thamnophis sirtalis) in Pennsylvania. J Zoo Wildlife Med 47:341–346. https://doi.org/10.1638/2015-0123.1
Origgi FC, Tecilla M (2020) Immunology of reptiles. In: Jacobson ER, Garner MM (Eds.) Infectious diseases and pathology of reptiles: color atlas and text, diseases and pathology of reptiles Volume 1, 2nd edn. CRC Press, Florida, pp 215–266
Paré JA, Sigler L, Rypien KL, Gibas CFC (2003) Cutaneous mycobiota of captive squamate reptiles with notes on the scarcity of Chrysosporium anamorph of Nannizziopsis vriesii. J Herpetol Med Surg 13:10–15. https://doi.org/10.5818/1529-9651.13.4.10
Paré JA, Sigler L (2016) An overview of reptile fungal pathogens in the genera Nannizziopsis, Paranannizziopsis, and Ophidiomyces. J Herpetol Med Surg 26:46–53. https://doi.org/10.5818/1529-9651-26.1-2.46
Paré JA, Conley KJ (2020) Mycotic Diseases of Reptiles. In: Jacobson ER, Garner MM (eds) Infectious diseases and pathology of reptiles: color atlas and text. CRC Press Boca Raton, Florida, USA, pp 795–857
Paré JA, Wellehan J, Perry SM, Scheelings TF, Keller K, Boyer T (2020) Onygenalean dermatomycoses (formerly yellow fungus disease, snake fungal disease) in reptiles. J Herpetol Med Surg 30:198–209. https://doi.org/10.5818/19-12-221.1
Park ST, Collingwood AM, St-Hilaire S, Sheridan PP (2014) Inhibition of Batrachochytrium dendrobatidis caused by bacteria isolated from the skin of boreal toads, Anaxyrus (Bufo) boreas, from Grand Teton National Park, Wyoming, USA. Microbiol Insights 7:1. https://doi.org/10.4137/MBI.S13639
Patel S, Meixner JA, Smith MB, Mcginnis MR (2017) Superficial mycoses and dermatophytes. In: Tyring SK, Lupi O, Hengge UR (Eds.) Tropical dermatology, 2nd edn. Elsevier, Netherlands, pp 189–201. https://doi.org/10.1016/B978-0-323-29634-2.00017-1
Patterson JR, Bender MJ, Duckworth CE, Noble E, Patterson DB, Pilgrim Z (2021) The occurrence of Ophidiomyces ophiodiicola in Northern Georgia wild and captive snake populations. J Wildl Dis 57:643–647. https://doi.org/10.7589/JWD-D-20-00129
Persons TB, Stengle AG, Yorks DT, Demaynadier PG (2017) Snake Fungal Disease from Maine, USA, and an Update of Documented Occurrences in the United States. Herpetol Rev 48:62–63
Peterson NR, Rose K, Shaw S, Hyndman TH, Sigler L, Kurtböke Dİ, Frère C (2020) Cross-continental emergence of Nannizziopsis barbatae disease may threaten wild Australian lizards. Sci Rep-Uk 10:1–12. https://doi.org/10.1038/s41598-020-77865-7
Picquet P, Heckers KO, Kolesnik E, Heusinger A, Marschang RE (2018) Detection of Ophidiomyces ophiodiicola in two captive bocourt water snakes (Subsessor bocourti) and one captive pueblan milk snake (Lampropeltis triangulum campbelli). J Zoo Wildlife Med 49:219–222. https://doi.org/10.1638/2017-0112R.1
Pohly AE (2020) Pathological characterization of natural Ophidiomyces ophiodiicola infection in wild-caught Lake Erie watersnakes: a standardized approach to documentation of disease. Master’s thesis, University of Illinois at Urbana-Champaign, Urbana, Illinois
Price SJ, Oldham CR, Boys WM, Fleckenstein LJ (2015) First record of snake fungal disease in Kentucky. JKAS 76:47–48. https://doi.org/10.3101/1098-7096-76.1.47
Rajeev S, Sutton DA, Wickes BL, Miller DL, Giri D, Van Meter M, Guarro J (2009) Isolation and characterization of a new fungal species, Chrysosporium ophiodiicola, from a mycotic granuloma of a black rat snake (Elaphe obsoleta obsoleta). J Clin Microbiol 47:1264–1268. https://doi.org/10.1128/JCM.01751-08
Ratnasingham S, Hebert PD (2007) Bold: The Barcode of Life Data System (http://www.barcodinglife.org). Mol Ecol Notes 7:355–364. https://doi.org/10.1111/j.1471-8286.2007.01678.x. http://www.boldsystems.org/index.php/Taxbrowser_Taxonpage?searchMenu=taxonomy&query=Ophidiomyces+ophiodiicola&taxon=Ophidiomyces+ophiodiicola. Accessed 20 July 2021
Ravesi MJ, Tetzlaff SJ, Allender MC, Kingsbury BA (2016) Detection of snake fungal disease from a Lampropeltis triangulum (eastern milksnake) in northern Michigan. Northeast Nat. https://doi.org/10.1656/045.023.0310
Regester KJ, Allender MC, Hayward K (2017) Surveillance for the snake fungal disease pathogen Ophidiomyces ophiodiicola in the Eastern Massasauga Rattlesnake (Sistrurus c. catenatus), Pennsylvania, USA. Herpetol Rev 48:772
Reynolds H, Raudabaugh D, Lilje O, Allender M, Miller AN, Gleason F (2017) Chapter 27: Emerging mycoses and fungus-like diseases of vertebrate wildlife. In: Dighton J, White JF (eds) The fungal community: its organization and role in the ecosystem. CRC Press, Florida, pp 286–403
Rivera S (2019) Quarantine. In: Divers SJ, Stahl S (eds) Mader’s reptile and amphibian medicine and surgery, 3rd edn. Elsevier Health Sciences, St. Louis, Missouri, pp 142–144
Robert V, Vu D, Amor ABH, van de Wiele N, Brouwer C, Jabas B, Szoke S, Dridi A, Triki M, ben Daoud S, Chouchen O, Vaas L, de Cock A, Stalpers JA, Stalpers D, Verkley GJM, Groenewald M, Borges dos Santos F, Stegehuis G, Li W, Wu L, Zhang R, Ma J, Zhou M, Pérez Gorjón S, Eurwilaichitr L, Ingsriswang S, Hansen K, Schoch C, Robbertse B, Irinyi L, Meyer W, Cardinali G, Hawksworth DL, Taylor JW, Crous PW (2013) MycoBank gearing up for new horizons. IMA fungus 4:371–379. https://doi.org/10.5598/imafungus.2013.04.02.16https://www.mycobank.org/page/Name%20details%20page/name/Ophidiomyces%20ophiodiicola. Accessed 20 July 2021
Robertson J, Chinnadurai SK, Woodburn DB, Adkesson MJ, Landolfi JA (2016) Disseminated Ophidiomyces ophiodiicola infection in a captive eastern massasauga (Sistrurus catenatus catenatus). J Zoo Wildlife Med 47:337–340. https://doi.org/10.1638/2014-0222.1
Romani L (2011) Immunity to fungal infections. Nat Rev Immunol 11:275–288. https://doi.org/10.1038/nri2939
Romani L, Puccetti P (2006) Protective tolerance to fungi: the role of IL-10 and tryptophan catabolism. Trends Microbiol 14:183–189. https://doi.org/10.1016/j.tim.2006.02.003
Rossi JV (2019) General husbandry and management. In: Divers SJ, Stahl S (eds) Mader’s reptile and amphibian medicine and surgery, 3rd edn. Elsevier Health Sciences, St. Louis, Missouri, pp 109–130
Round JL, Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9:313–323. https://doi.org/10.1038/nri2515
Rzadkowska M, Allender MC, O’Dell M, Maddox C (2016) Evaluation of common disinfectants effective against Ophidiomyces ophiodiicola, the causative agent of snake fungal disease. J Wildl Dis 52:759–762. https://doi.org/10.7589/2016-01-012
Scapigliati G, Buonocore F, Mazzini M (2006) Biological activity of cytokines: an evolutionary perspective. Curr Pharm Des 12:3071–3082. https://doi.org/10.2174/138161206777947489
Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Karsch-Mizrachi I (2020) NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database, 2020. https://doi.org/10.1093/database/baaa062 . https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=1387563&lvl=3&lin=f&keep=1&srchmode=1&unlock . Accessed 20 July 2021
Sigler L, Hambleton S, Paré JA (2013) Molecular characterization of reptile pathogens currently known as members of the Chrysosporium anamorph of Nannizziopsis vriesii complex and relationship with some human-associated isolates. J Clin Microbiol 51:3338–3357. https://doi.org/10.1128/JCM.01465-13
Sigler L (2013) Ophidiomyces Sigler L, Hamleton S, Paré JA, gen. nov. IF550166 andIF550167. Nomenclatural novelties: Lynne Sigler. Index Fungorum no. 19. http://www.indexfungorum.org/Publications/Index%20Fungorum%20no.19.pdf. Accessed 17 May 2021
Sleeman J (2013) Snake fungal disease in the United States. National Wildlife Health Center Wildlife Health Bulletin, 2013- 02. USGS
Smaga CR, Allender MC, Jiménez FA (2021) Estimation of prevalence and qPCR copy-number of Ophidiomyces ophiodiicola and snake fungal disease in a snake community in Southern Illinois, with Notes. Herpetol Rev 52:45–48
Smeekens SP, Huttenhower C, Riza A, Van de Veerdonk FL, Zeeuwen PL, Schalkwijk J, Van der Meer JW, Xavier RJ, Netea MG, Gevers D (2014) Skin microbiome imbalance in patients with STAT1/STAT3 defects impairs innate host defense responses. J Innate Immun 6:253–262. https://doi.org/10.1159/000351912
Smeenk NA, Lipps GJ, Backus K, Freeman M, Hribar D, Parsley M (2016) Snake fungal disease, Ophidiomyces ophiodiicola. Ohio, USA. Herpetol Rev 47:592–594
Smith CE, Edwards J, Lorch JM (2013) Crotalus horridus (timber rattlesnake) fungal pathogens. Herpetol Rev 44:519–520
Snyder SD, Sutton WB, Walker DM (2020) Prevalence of Ophidiomyces ophiodiicola, the causative agent of snake fungal disease, in the interior plateau ecoregion of Tennessee, USA. J Wildlife Dis 56:907–911. https://doi.org/10.7589/2019-04-109
Sperry JH, Wolff PJ, Melder CA, Nevarez JG, Huskins SD, Pearce SE (2021) Habitat use, activity patterns, and survival of Louisiana pinesnakes (Pituophis ruthveni) in West-central Louisiana. Southeast Nat 20:273–292. https://doi.org/10.1656/058.020.0206
Stchigel AM, Sutton DA, Cano-Lira JF, Cabañes FJ, Abarca L, Tintelnot K, Wickes BL, García D, Guarro J (2013) Phylogeny of chrysosporia infecting reptiles: proposal of the new family Nannizziopsiaceae and five new species. Pers Mol Phylogeny Evol Fungi 31: 86. https://doi.org/10.3767/003158513x669698
Steeil JC, Hope KL, Evans M, Peters A, Cartoceti A (2018a) Multifocal Ophidiomyces ophiodiicola infection in an eastern diamondback rattlesnake (Crotalus adamanteus) without the presence of skin lesions. J Herpetol Med Surg 28:76–80. https://doi.org/10.5818/18-02-146.1
Steeil JC, Neiffer DL, Evans M, Peters A, Allender MC, Cartoceti A (2018b) Retrospective review of snake fungal disease (Ophidiomyces ophiodiicola) at the Smithsonian’s National Zoological Park (1983- 2017). Proceedings of the Joint AAZV and EAZWV, Prague, Czech Republic
Stegen G, Pasmans F, Schmidt BR, Rouffaer LO, Van Praet S, Schaub M, Canessa S, Laudelout A, Kinet T, Adriaensen C, Haesebrouck F, Bert W, Bossuyt F, Martel A (2017) Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 544:353–356. https://doi.org/10.1038/nature22059
Stengle AG, Farrell TM, Freitas KS, Lind CM, Price SJ, Butler BO, Tadevosyan T, Isidoro-Ayza M, Taylor DR, Winzeler M, Lorch JM (2019) Evidence of vertical transmission of the snake fungal pathogen Ophidiomyces ophiodiicola. J Wildlife Dis 55:961–964. https://doi.org/10.7589/2018-10-250
Strand DA, Jussila J, Johnsen SI, Viljamaa-Dirks S, Edsman L, Wiik-Nielsen J, Vrålstad T (2014) Detection of crayfish plague spores in large freshwater systems. J Appl Ecol 51:544–553. https://doi.org/10.1111/1365-2664.12218
Sun PL, Yang CK, Li WT, Lai WY, Fan YC, Huang HC, Yu PH (2021) Infection with Nannizziopsis guarroi and Ophidiomyces ophiodiicola in reptiles in Taiwan. Transbound Emerg Dis. https://doi.org/10.1111/tbed.14049
Takami Y, Nam KO, Takaki Y, Kadekaru S, Hemmi C, Hosoya T, Une Y (2021) First report of ophidiomycosis in Asia caused by Ophidiomyces ophiodiicola in captive snakes in Japan. J Vet Med Sci. https://doi.org/10.1292/jvms.21-0177
Terrell KA, Ballmann AE, Brown A, Childers C, Knowles S, Meredith A, Sparks D (2020) Environmental contamination and unusual snake mortality in an urban national wildlife refuge. Herpetol Conserv Bio 15:652–665
Tetzlaff SJ, Allender M, Ravesi M, Smith J, Kingsbury B (2015) First report of snake fungal disease from Michigan, USA involving Massasaugas, Sistrurus catenatus (Rafinesque 1818). Herpetol Notes 8:31–33
Tetzlaff SJ, Ravesi MJ, Allender MC, Carter ET, DeGregorio BA, Josimovich JM, Kingsbury BA (2017) Snake fungal disease affects behavior of free-ranging massasauga rattlesnakes (Sistrurus catenatus). Herpetol Conserv Bio 12:624–634
Todd G, Jodrey A, Stahlschmidt Z (2016) Immune activation influences the trade-off between thermoregulation and shelter use. Anim Behav 118:27–32. https://doi.org/10.1016/j.anbehav.2016.05.003
Turner FB (1978) The dynamics of populations of squamates, crocodilians and rhynchocephalians. In: Gans C, Tinkle DW (Eds.) Biology of the Reptilia. Volume 7. Ecology and Behaviour, pp 157–264
Turner GG, Meteyer CU, Barton H, Gumbs JF, Reeder DM, Overton B, Bandouchova H, Bartonička T, Martínková N, Pikula J, Zukal J, Blehert DS (2014) Nonlethal screening of bat-wing skin with the use of ultraviolet fluorescence to detect lesions indicative of white-nose syndrome. J Wildlife Dis 50:566–573. https://doi.org/10.7589/2014-03-058
TWRA (2017) Snake fungal disease monitoring report for Tennessee. Tennessee Wildlife Resources Agency. https://www.tn.gov/content/dam/tn/twra/documents/reptiles/Snake-Fungal-Disease-Monitoring-Report_2012-2017.pdf. Accessed 6 April 2021
USFWS (2018) Species status assessment (SSA) report for the Eastern Indigo Snake (Drymarchon couperi), Version 1.0 November 2018. U.S. Fish and Wildlife Service. Atlanta, GA. https://ecos.fws.gov/ServCat/DownloadFile/157073. Accessed 10 February 2022
Veilleux J, Dombrowski DS, Allender MC, Lewbart G (2020) Diagnosis, treatment and post-release monitoring of an eastern black rat snake (Pantherophis alleghaniensis) with ophidiomycosis and traumatic injuries. Vet Rec Case Rep 8:e000954. https://doi.org/10.1136/vetreccr-2019-000954
Vissiennon T, Schüppel KF, Ullrich E, Kuijpers AF (1999) Case report. A disseminated infection due to Chrysosporium queenslandicum in a garter snake (Thamnophis). Mycoses 42:107–110. https://doi.org/10.1046/j.1439-0507.1999.00409.x
Vivirito K, Haynes E, Adamovicz L, Wright A, Durante K, Stanford K, Allender M (2021) Ultraviolet fluorescence as a field-applicable screening tool for lesions consistent with Ophidiomycosis in Lake Erie watersnakes (Nerodia sipedon insularum). J Wildlife Dis 57:380–385. https://doi.org/10.7589/JWD-D-20-00013
Walden HD, Iredale ME, Childress A, JrJF W, Ossiboff RJ (2020) Case report: invasive pentastomes, Raillietiella orientalis (Sambon, 1922), in a free-ranging banded water snake (Nerodia fasciata) in North Central Florida, USA. Front Vet Sci. https://doi.org/10.3389/fvets.2020.00467
Walker DM, Leys JE, Grisnik M, Grajal-Puche A, Murray CM, Allender MC (2019) Variability in snake skin microbial assemblages across spatial scales and disease states. ISME J 13:2209–2222. https://doi.org/10.1038/s41396-019-0416-x
Wang H, Ye J (2015) Regulation of energy balance by inflammation: common theme in physiology and pathology. Rev Endocr Metab Disord 16:47–54. https://doi.org/10.1007/s11154-014-9306-8
Ward RJ, Griffiths RA, Wilkinson JW, Cornish N (2017) Optimising monitoring efforts for secretive snakes: a comparison of occupancy and N-mixture models for assessment of population status. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-18343-5
Waugh L, Ramachandran A, Talent S, Cole G, D’Agostino J (2017) Survey of aerobic and anaerobic blood cultures in free-ranging Western Ratsnakes (Pantherophis obsoletus). J Herpetol Med Surg 27:44–47. https://doi.org/10.5818/1529-9651-27.1-2.44
Wittwer C, Stoll S, Strand D, Vrålstad T, Nowak C, Thines M (2018) eDNA-based crayfish plague monitoring is superior to conventional trap-based assessments in year-round detection probability. Hydrobiologia 807:87–97. https://doi.org/10.1007/s10750-017-3408-8
Woodburn DB, Miller AN, Allender MC, Maddox CW, Terio KA (2019) Emydomyces testavorans, a new genus and species of onygenalean fungus isolated from shell lesions of freshwater aquatic turtles. J Clin Microbiol 57:e00628-e718. https://doi.org/10.1128/JCM.00628-18
Woodhams DC, Brandt H, Baumgartner S, Kielgast J, Ku¨pfer E, Tobler U, Davis LR, Schmidt BR, Bel C, Hodel S, Knight R, McKenzie V (2014) Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS ONE 9:e96375. https://doi.org/10.1371/journal.pone.0096375
Zaher H, Murphy RW, Arredondo JC, Graboski R, Machado-Filho PR, Mahlow K, Montingelli GG, Bottallo Quadros A, Orlov NL, Wilkinson M, Zhang YP, Grazziotin FG (2019) Large-scale molecular phylogeny, morphology, divergence-time estimation, and the fossil record of advanced caenophidian snakes (Squamata: Serpentes). PLoS ONE 14:e0216148. https://doi.org/10.1371/journal.pone.0216148
Zimmerman LM, Vogel LA, Bowden RM (2010) Understanding the vertebrate immune system: insights from the reptilian perspective. J Exp Biol 213:661–671. https://doi.org/10.1242/jeb.038315
Acknowledgements
We are grateful to Lynne Sigler, UAMH Centre for Global Microfungal Biodiversity, Becki Lawson (Institute of Zoology, Zoological Society of London), Steven J. R. Allain (Durrell Institute of Conservation and Ecology, University of Kent) and Grégoire Meier for providing us with their images. We would like to thank the anonymous reviewers who improved the quality of the manuscript. We acknowledge Enrica Calò, Anna Cerullo, Veronica Crocchianti, and Marcelo Martínez-Barbitta for a pre-review of the manuscript. We thank Leah Cannon her useful comments on pathology and for the English language editing of this manuscript.
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
Conceptualization: Matteo Riccardo Di Nicola and Daniele Marini; literature search and data analysis: Matteo Riccardo Di Nicola, Luca Coppari, Tommaso Notomista, and Daniele Marini; writing–original draft preparation: Matteo Riccardo Di Nicola, Luca Coppari, Tommaso Notomista, and Daniele Marini; writing–review and editing: Matteo Riccardo Di Nicola and Daniele Marini.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Table showing all the ophidian species in which the presence of the fungus has been detected. For each species, the following data are listed: the case classification based on the present review (see “Materials and methods” section for further information), the geographical locations, the bibliographic references, and the threat level/population trend according to the IUCN red list. For each species, the “Classification” column lists the highest classification rank that at least one specimen has reached. The ranking followed this increasing order: (i) “Oo present”; (ii) “apparent ophidiomycosis”; (iii) “Ophidiomycosis”; (iv) “Ophidiomycosis and Oo shedder.” Only the specimens (and related references) suitable to be ranked in one of these case classifications have been included in Appendix 1.
Family | Species | States | Classification | References | IUCN | Trend |
|---|---|---|---|---|---|---|
Acrochordidae | Acrochordus sp. | Australiaa | Apparent ophidiomycosis | Sigler et al. (2013) | / | / |
Boidae | Chilabothrus inornatus | Puerto Rico | Oo present | Allender et al. (2020) | LC | Unknown |
Corallus hortulana | WAa | Oo present | Steeil et al. (2018b) | LC | Stable | |
Eunectes murinus | CAa, WAa | Ophidiomycosis and Oo Shedder | Bicknese (2009); (Sigler et al. 2013); Steeil et al. (2018b) | NE | / | |
Eunectes notaeus | WAa | Oo present | Steeil et al. (2018b) | NE | / | |
Colubridae | Boiga irregularis | MDa | Ophidiomycosis and Oo Shedder | LC | Stable | |
Cemophora coccinea | FL, GA | Ophidiomycosis and Oo Shedder | LC | Stable | ||
Coluber constrictor | CT, FL, GA, IL, KY, LA, MD, MS, NY, OH, PAa, TN, VA, n.d | Ophidiomycosis and Oo Shedder | Allender et al. (2020); Fuchs et al. (2020); Grisnik et al. (2018); Guthrie et al. (2016); Haynes et al. (2020); Hill et al. (2018); Licitra et al. (2019); Long et al. (2019); Lorch et al. (2016b); McKenzie et al. (2019); Ohkura et al. (2016); Patterson et al. (2021); Smaga et al. (2021); Terrell et al. (2020); TWRA (2017) | LC | Stable | |
Drymarchon couperi | GA, n.d | Apparent ophidiomycosis | LC | Decreasing | ||
Lampropeltis californiae | CA, GAa | Ophidiomycosis and Oo Shedder | LC | Stable | ||
Lampropeltis elapsoides | FL | Ophidiomycosis | Guzman-Vargas et al. (2020) | LC | Stable | |
Lampropeltis getula | FL, GAb, KY, MD, NC, TN, VA | Apparent ophidiomycosis | Fuchs et al. (2020); Glorioso et al. (2020); Grisnik et al. (2018); Haynes et al. (2020); McKenzie et al. (2019); Patterson et al. (2021); Stengle et al. (2019); TWRA (2017) | LC | Stable | |
Lampropeltis nigra | AL, IL, KY, TN, n.d | Apparent ophidiomycosis | Grisnik et al. (2018); Lorch et al. (2016b); Paré and Conley (2020); Smaga et al. (2021); Snyder et al. (2020); TWRA (2017) | LC | Stable | |
Lampropeltis triangulum | Francea, KY, MA, MI, NH, NY, TN, WAa, WI, n.d | Ophidiomycosis | Allender et al. (2020); Grisnik et al. (2018); Lorch et al. (2016b); McKenzie et al. (2019); Paré and Conley (2020); Picquet et al. (2018); Ravesi et al. (2016); Steeil et al. (2018b); Stengle et al. (2019); TWRA (2017) | LC | Stable | |
Dinodon rufozonatum | Taiwan | Ophidiomycosis and Oo Shedder | Sun et al. (2021) | LC | Stable | |
Masticophis flagellum | TX | Oo present | Lizarraga (2020) | LC | Stable | |
Opheodrys aestivus | GA, n.d | Apparent ophidiomycosis | LC | Stable | ||
Pantherophis alleghaniensis | CT, GA, MD, NJ, NC, PA, VT, VA, n.d | Ophidiomycosis and Oo Shedder | Allender et al. (2020); Fuchs et al. (2020); Haynes et al. (2020); Licitra et al. (2019); Lorch et al. (2016b); Paré and Conley (2020); Rajeev et al. (2009); Regester et al. (2017); Veilleux et al. (2020) | LC | Unknown | |
Pantherophis emoryi | n.d | Oo present | Allender et al. (2020) | LC | Stable | |
Pantherophis guttatus | GAb, NY, TN, n.d | Ophidiomycosis and Oo Shedder | Grisnik et al. (2018); Haynes et al. (2020); Patterson et al. (2021); Paré and Conley (2020); Sigler et al. (2013); TWRA (2017) | LC | Stable | |
Pantherophis obsoletus* | Japana, GAb, TN, TX, n.d | Ophidiomycosis and Oo Shedder | Allender et al. (2020); Lizarraga (2020); Patterson et al. (2021); Rajeev et al. (2009)c; Takami et al. (2021); TWRA (2017) | LC | Unknown | |
Pantherophis ramspotti | n.d | Oo present | Allender et al. (2020) | LC | Stable | |
Pantherophis sp. | WI | Oo present | Lorch et al. (2016b) | / | / | |
Pantherophis spiloides | Ontario (Canada), GA, IL, KY, OH, TN, n.d | Ophidiomycosis and Oo Shedder | Allender et al. (2020); Davy et al. (2021); Grisnik et al. (2018); Long et al. (2019); Lorch et al. (2021); McKenzie et al. (2019); Patterson et al. (2021); Smaga et al. (2021) | LC | Unknown | |
Pantherophis vulpinus** | Ontario (Canada), WI | Apparent ophidiomycosis | CWHC (2017); Davy et al. (2021); Dillon (2020); Lorch et al. (2016b); TWRA (2017) | LC | Stable | |
Pituophis catenifer | MN, n.d | Oo present | LC | Stable | ||
Pituophis melanoleucus | FL, GA, n.d | Ophidiomycosis | Guzman-Vargas et al. (2020); Haynes et al. (2020); Paré and Conley (2020) | LC | Decreasing | |
Pituophis ruthveni | LA | Oo present | EN | Decreasing | ||
Storeria dekayi | Ontario (Canada), CT, MD, TN, VA | Apparent ophidiomycosis | Davy et al. (2021); Dillon (2020); Fuchs et al. (2020); Grisnik et al. (2018); Licitra et al. (2019); TWRA (2017) | LC | Stable | |
Storeria occipitomaculata | KY | Apparent ophidiomycosis | McKenzie et al. (2019) | LC | Stable | |
Dipsadidae | Carphophis amoenus | GA, MD, KY, VA, n.d | Apparent ophidiomycosis | Allender et al. (2020); Fuchs et al. (2020); McKenzie et al. (2019); Patterson et al. (2021) | LC | Stable |
Diadophis punctatus | GA, KY, MD, TN, VA, n.d | Apparent ophidiomycosis | Allender et al. (2020); Fuchs et al. (2020); Grisnik et al. (2018); Haynes et al. (2020); McKenzie et al. (2019) | LC | Stable | |
Farancia abacura | FL, GA | Ophidiomycosis and Oo Shedder | Guzman-Vargas et al. (2020); Haynes et al. (2020); Last et al. (2016); Lorch et al. (2016b) | LC | Stable | |
Farancia erytrogramma | GA, VA | Ophidiomycosis and Oo Shedder | LC | Stable | ||
Heterodon platirhinos | GA, OH, TN | Apparent ophidiomycosis | Grisnik et al. (2018); Haynes et al. (2020); Smeenk et al. (2016); TWRA (2017) | LC | Stable | |
Hydrodynastes gigas | WAa | Oo present | Steeil et al. (2018b) | NE | / | |
Elapidae | Hoplocephalus bungaroides | Australiaa | Ophidiomycosis and Oo Shedder | McLelland et al. (2010); (Sigler et al. 2013); McLelland pers. comm.c | VU | Unknown |
Naja atra | Taiwan | Ophidiomycosis and Oo Shedder | Sun et al. (2021) | VU | Decreasing | |
Homalopsidae | Subsessor bocourti | Francea | Ophidiomycosis | Picquet et al. (2018) | LC | Unknown |
Natricidae | Natrix helvetica*** | UK, Switzerland | Ophidiomycosis and Oo Shedder | LC | Decreasing | |
Natrix tessellata | Czech Republic | Apparent ophidiomycosis | Franklinos et al. (2017) | LC | Decreasing | |
Nerodia clarkii | FLb | Ophidiomycosis | LC | Decreasing | ||
Nerodia erythrogaster | GA, IL, KY, TN, n.d | Apparent ophidiomycosis | Allender et al. (2020); Grisnik et al. (2018); Haynes et al. (2020); McKenzie et al. (2019); Smaga et al. (2021); TWRA (2017) | LC | Stable | |
Nerodia fasciata | FL, GA, LA, n.d | Ophidiomycosis and Oo Shedder | Allender et al. (2020); Glorioso et al. (2016); Haynes et al. (2020); Walden et al. (2020) | LC | Stable | |
Nerodia rhombifer | n.d | Oo present | Allender et al. 2020 | LC | Stable | |
Nerodia sipedon | Ontario (Canada), CT, KY, MD, ME, MA, MO, OH, TN, VA, n.d | Ophidiomycosis and Oo Shedder | Allender et al. (2020); Baker et al. (2019); CWHC (2017); Davy et al. (2021); Dibadj et al. (2021); Dillon (2020); Fredrickson (2019); Fuchs et al. (2020); Grisnik et al. (2018); Guthrie et al. (2016); Licitra et al. (2019); Lorch et al. (2016b); McKenzie et al. (2019); McKenzie et al. (2020a, b); Persons et al. (2017); Pohly (2020); Snyder et al. (2020); Vivirito et al. (2021); TWRA (2017) | LC | Stable | |
Nerodia taxispilota | FL, GA, VA | Ophidiomycosis | Guthrie (2016); Guthrie et al. (2016); Guzman-Vargas et al. (2020); Haynes et al. (2020) | LC | Stable | |
Regina septemvittata | Ontario (Canada), KY, TN | Ophidiomycosis | CWHC (2017); Davy et al. (2021); Grisnik et al. (2018); Lorch et al. (2016b); McKenzie et al. (2019); McKenzie et al. (2020a, b); Price et al. (2015); Stengle et al. (2019); TWRA (2017) | LC | Stable | |
Thamnophis proximus | LA, TX | Oo present | LC | Stable | ||
Thamnophis radix | IL | Ophidiomycosis and Oo Shedder | Dolinski et al. (2014) | LC | Stable | |
Thamnophis saurita | Ontario (Canada), GA, MD, TN, VA, n.d | Apparent ophidiomycosis | Allender et al. (2020); Davy et al. (2021); Fuchs et al. (2020); Grisnik et al. (2018); Haynes et al. (2020); TWRA (2017) | LC | Stable | |
Thamnophis sirtalis | Ontario (Canada), CT, GA, IL, KY, NY, OH, PAa, TN, n.d | Ophidiomycosis | CWHC (2017); Davy et al. (2021); Grisnik et al. (2018); Haynes et al. (2020); Lorch et al. (2016b); McKenzie et al. (2019); McKenzie et al. (2020a, b); Price et al. (2015); Smaga et al. (2021); Stengle et al. (2019); TWRA (2017) | LC | Stable | |
Thamnophis sp. | Germaniaa | Ophidiomycosis and Oo Shedder | / | / | ||
Virginia valeriae | KY, TN | Oo present | Lorch et al. (2016b); McKenzie et al. (2019); Snyder et al. (2020) | LC | Stable | |
Pythonidae | Python bivittatus | FL | Oo present | Glorioso et al. (2020) | VU | Decreasing |
Python regius | UKa | Ophidiomycosis | Sigler et al. (2013) | LC | Unknown | |
Python sebae | FLa | Oo present | NE | / | ||
Viperidae | Agkistrodon contortrix | GA, KY, SC, TN, TX, n.d | Apparent ophidiomycosis | Allender et al. (2020); Haynes et al. (2020); Lizarraga (2020); Lorch et al. (2016b); McKenzie et al. (2019); Paré and Conley (2020); Snyder et al. (2020); TWRA (2017) | LC | Stable |
Agkistrodon piscivorus | FL, GA, LA, MS, TN, n.d | Apparent ophidiomycosis | Allender et al. (2020); Grisnik et al. (2018); Haynes et al. (2020); Lorch et al. (2016b); Terrell et al. (2020) | LC | Stable | |
Crotalus adamanteus | FL, GA, WAa, n.d | Ophidiomycosis | Allender et al. (2020); Glorioso et al. (2020); Haynes et al. (2020); Steeil et al. (2018a, b) | LC | Decreasing | |
Crotalus horridus | CT, GA, IL, KY, MA, MN, NH, NY, PA, TN, VT, WI, n.d | Ophidiomycosis and Oo Shedder | Britton et al. (2019); Dibadj et al. (2021); Grisnik et al. (2018); Haynes et al. (2020); Hill et al. (2018); Januszkiewicz et al. (2020); Lorch et al. (2016b), (2021); McKenzie et al. (2019); Smith et al. (2013); TWRA (2017) | LC | Decreasing | |
Sistrurus catenatus | Ontario (Canada), ILb, MA, MI, n.d | Ophidiomycosis and Oo Shedder | Allender et al. (2011, 2015a, 2016, 2018, 2020); Baker et al. (2019); Britton et al. (2019); CWHC (2017); Davy et al. (2021); Hileman et al. (2018); Lorch et al. (2016b); McBride et al. (2015); Robertson et al. (2016); Tetzlaff et al. (2015, 2017) | LC | Stable | |
Sistrurus miliarius | FL; GA | Ophidiomycosis | Agugliaro et al. (2020); Guzman-Vargas et al. (2020); Haynes et al. (2020); Lind et al. (2018a, 2019); Lorch et al. (2016b); Stengle et al. (2019) | LC | Stable | |
Vipera berus | UK | Oo present | Franklinos et al. (2017) | LC | Decreasing |
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Nicola, M.R., Coppari, L., Notomista, T. et al. Ophidiomyces ophidiicola detection and infection: a global review on a potential threat to the world’s snake populations. Eur J Wildl Res 68, 64 (2022). https://doi.org/10.1007/s10344-022-01612-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-022-01612-8