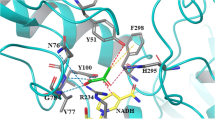
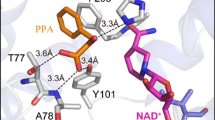
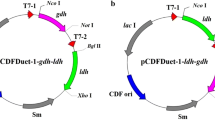
Abstract
Lactoyl-CoA is critical for the biosynthesis of biodegradable and biocompatible lactate-based copolymers, which have wide applications. However, reports on acetyl-CoA: lactate CoA-transferases (ALCTs) are rare. To exploit novel ALCTs, amino acid sequence similarity searches based on the CoA-transferases from Clostridium propionicum and Megasphaera elsdenii were conducted. Two known and three novel enzymes were expressed, purified and characterized. Three novel ALCTs were identified, one each from Megasphaera sp. DISK 18, Clostridium lactatifermentans An75 and Firmicutes bacterium CAG: 466. ME-PCT from Megasphaera elsdenii had the highest catalytic efficiency for both acetyl-CoA (264.22 s−1 mM−1) and d-lactate (84.18 s−1 mM−1) with a broad temperature range for activity and good stability. This study, therefore, offers novel and efficient enzymes for lactoyl-CoA generation. To our best knowledge, this is the first report on the systematic mining of ALCTs, which offers valuable new tools for the engineering of pathways that rely on these enzymes.







Similar content being viewed by others
References
Boynton ZL, Bennett GN, Rudolph FB (1994) Intracellular concentrations of coenzyme A and its derivatives from Clostridium acetobutylicum ATCC 824 and their roles in enzyme regulation. Appl Environ Microbiol 60:39–44
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Buckel W, Bobi A (1976) The enzyme complex citramalate lyase from Clostridium tetanomorphum. Eur J Biochem 64:255–262. https://doi.org/10.1111/j.1432-1033.1976.tb10295.x
Buckel W, Dorn U, Semmler R (1981) Glutaconate CoA-transferase from Acidaminococcus fermentans. Eur J Biochem 118:315–321. https://doi.org/10.1111/j.1432-1033.1981.tb06404.x
Chen Y, Nielsen J (2016) Biobased organic acids production by metabolically engineered microorganisms. Curr Opin Biotechnol 37:165–172. https://doi.org/10.1016/j.copbio.2015.11.004
Choi SY, Park SJ, Kim WJ, Yang JE, Lee H, Shin J, Lee SY (2016) One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli. Nat Biotechnol 34:435–440. https://doi.org/10.1038/nbt.3485
Counotte GH, Prins RA, Janssen RH, Debie MJ (1981) Role of Megasphaera elsdenii in the fermentation of DL-[2-13C]lactate in the rumen of dairy cattle. Appl Environ Microbiol 42:649–655
Danner H, Urmos M, Gartner M, Braun R (1998) Biotechnological production of acrylic acid from biomass. Appl Biochem Biotechnol 70–72:887–894. https://doi.org/10.1007/bf02920199
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Garg S, Clomburg JM, Gonzalez R (2018) A modular approach for high-flux lactic acid production from methane in an industrial medium using engineered Methylomicrobium buryatense 5GB1. J Ind Microbiol Biotechnol 45:379–391. https://doi.org/10.1007/s10295-018-2035-3
Gokarn RR, Selifonova OV, Jessen HJ, Gort SJ, Selmer T, Buckel W (2009) 3-Hydroxypropionic acid and other organic compounds. Reexamination certificate second reexamination - United States. Application: US20070942669 on 2007-11-19. US7638316, 29 Dec 2009. http://europepmc.org/patents/PAT/US7638316
Heider J (2001) A new family of CoA-transferases. FEBS Lett 509:345–349. https://doi.org/10.1016/S0014-5793(01)03178-7
Hishinuma F, Kanegasaki S, Takahashi H (1968) Ruminal fermentation and sugar concentrations. Agric Biol Chem 32:1325–1330. https://doi.org/10.1080/00021369.1968.10859234
Jambunathan P, Zhang K (2016) Engineered biosynthesis of biodegradable polymers. J Ind Microbiol Biotechnol 43:1037–1058. https://doi.org/10.1007/s10295-016-1785-z
Jung YK, Kim TY, Park SJ, Lee SY (2010) Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol Bioeng 105:161–171. https://doi.org/10.1002/bit.22548
Kandasamy V, Vaidyanathan H, Djurdjevic I, Jayamani E, Ramachandran KB, Buckel W, Jayaraman G, Ramalingam S (2013) Engineering Escherichia coli with acrylate pathway genes for propionic acid synthesis and its impact on mixed-acid fermentation. Appl Microbiol Biotechnol 97:1191–1200. https://doi.org/10.1007/s00253-012-4274-y
Kumar S, Stecher G, Tamura KJMBE (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leutwein C, Heider J (2001) Succinyl-CoA: (R)-benzylsuccinate CoA-transferase: an enzyme of the anaerobic toluene catabolic pathway in denitrifying bacteria. J Bacteriol 183:4288–4295. https://doi.org/10.1128/jb.183.14.4288-4295.2001
Li N, Zhang B, Chen T, Wang Z, Y-j Tang, Zhao X (2013) Directed pathway evolution of the glyoxylate shunt in Escherichia coli for improved aerobic succinate production from glycerol. J Ind Microbiol Biotechnol 40:1461–1475. https://doi.org/10.1007/s10295-013-1342-y
Lindenkamp N, Schuermann M, Steinbuechel A (2013) A propionate CoA-transferase of Ralstonia eutropha H16 with broad substrate specificity catalyzing the CoA thioester formation of various carboxylic acids. Appl Microbiol Biotechnol 97:7699–7709. https://doi.org/10.1007/s00253-012-4624-9
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666. https://doi.org/10.1021/ja01318a036
Luo H, Zhou D, Liu X, Nie Z, Quiroga-Sanchez DL, Chang Y (2016) Production of 3-hydroxypropionic acid via the propionyl-CoA pathway using recombinant Escherichia coli strains. PLoS One 11:e0156286. https://doi.org/10.1371/journal.pone.0156286
Marounek M, Fliegrova K, Bartos S (1989) Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl Environ Microbiol 55:1570–1573
Prabhu R, Altman E, Eiteman MA (2012) Lactate and acrylate metabolism by Megasphaera elsdenii under batch and steady-state conditions. Appl Environ Microbiol 78:8564–8570. https://doi.org/10.1128/AEM.02443-12
Rajashekhara E, Hosoda A, Sode K, Ikenaga H, Watanabe K (2006) Volatile fatty acid-sensing system involving coenzyme-A transferase. Biotechnol Prog 22:334–337. https://doi.org/10.1021/bp050240p
Rangarajan ES, Li Y, Ajamian E, Iannuzzi P, Kernaghan SD, Fraser ME, Cygler M, Matte A (2005) Crystallographic trapping of the glutamyl-CoA thioester intermediate of family I CoA transferases. J Biol Chem 280:42919–42928. https://doi.org/10.1074/jbc.M510522200
Rochet J-C, Bridger WA (1994) Identification of glutamate 344 as the catalytic residue in the active site of pig heart CoA transferase. Protein Sci 3:975–981. https://doi.org/10.1002/pro.5560030613
Russell JB, Baldwin RL (1978) Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol 36:319–329
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schweiger G, Buckel W (1984) On the dehydration of (R)-lactate in the fermentation of alanine to propionate by Clostridium propionicum. FEBS Lett 171:79–84
Selmer T, Buckel W (1999) Oxygen exchange between acetate and the catalytic glutamate residue in glutaconate CoA-transferase from Acidaminococcus fermentans—Implications for the mechanism of CoA-ester hydrolysis. J Biol Chem 274:20772–20778. https://doi.org/10.1074/jbc.274.30.20772
Selmer T, Willanzheimer A, Hetzel M (2002) Propionate CoA-transferase from Clostridium propionicum—Cloning of the gene and identification of glutamate 324 at the active site. Eur J Biochem 269:372–380. https://doi.org/10.1046/j.0014-2956.2001.02659.x
Shozui F, Ki Matsumoto, Motohashi R, Sun J, Satoh T, Kakuchi T, Taguchi S (2011) Biosynthesis of a lactate (LA)-based polyester with a 96 mol% LA fraction and its application to stereocomplex formation. Polym Degrad Stab 96:499–504. https://doi.org/10.1016/j.polymdegradstab.2011.01.007
Taguchi S, Yamadaa M, Ki Matsumoto, Tajima K, Satoh Y, Munekata M, Ohno K, Kohda K, Shimamura T, Kambe H, Obata S (2008) A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc Natl Acad Sci USA 105:17323–17327. https://doi.org/10.1073/pnas.0805653105
Valdehuesa KN, Liu H, Nisola GM, Chung WJ, Lee SH, Park SJ (2013) Recent advances in the metabolic engineering of microorganisms for the production of 3-hydroxypropionic acid as C3 platform chemical. Appl Microbiol Biotechnol 97:3309–3321. https://doi.org/10.1007/s00253-013-4802-4
Volodina E, Schuermann M, Lindenkamp N, Steinbuechel A (2014) Characterization of propionate CoA-transferase from Ralstonia eutropha H16. Appl Microbiol Biotechnol 98:3579–3589. https://doi.org/10.1007/s00253-013-5222-1
Wang B, Zhang X, Yu X, Cui Z, Wang Z, Chen T, Zhao X (2019) Evolutionary engineering of Escherichia coli for improved anaerobic growth in minimal medium accelerated lactate production. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-018-09588-9
Werpy T, Petersen G, Aden A, Bozell J, Holladay J, White J, Manheim A, Eliot D, Lasure L, Jones S (2004) Top value added chemicals from biomass. Volume 1-Results of screening for potential candidates from sugars and synthesis gas. Department of Energy Washington DC, Washington DC
Wu Q, Liu T, Zhu L, Huang H, Jiang L (2017) Insights from the complete genome sequence of Clostridium tyrobutyricum provide a platform for biotechnological and industrial applications. J Ind Microbiol Biotechnol 44:1245–1260. https://doi.org/10.1007/s10295-017-1956-6
Yamada M, Ki Matsumoto, Nakai T, Taguchi S (2009) Microbial production of lactate-enriched poly (R)-lactate-co-(R)-3-hydroxybutyrate with novel thermal properties. Biomacromolecules 10:677–681. https://doi.org/10.1021/bm8013846
Yamada M, Ki Matsumoto, Shimizu K, Uramoto S, Nakai T, Shozui F, Taguchi S (2010) Adjustable mutations in lactate (LA)-polymerizing enzyme for the microbial production of LA-based polyesters with tailor-made monomer composition. Biomacromolecules 11:815–819. https://doi.org/10.1021/bm901437z
Yang TH, Kim TW, Kang HO, Lee S-H, Lee EJ, Lim S-C, Oh SO, Song A-J, Park SJ, Lee SY (2010) Biosynthesis of polylactic acid and its copolymers using evolved propionate CoA transferase and PHA synthase. Biotechnol Bioeng 105:150–160. https://doi.org/10.1002/bit.22547
Ye Z, Li X, Cheng Y, Liu Z, Tan G, Zhu F, Fu S, Deng Z, Liu T (2016) Evaluation of 3-hydroxypropionate biosynthesis in vitro by partial introduction of the 3-hydroxypropionate/4-hydroxybutyrate cycle from Metallosphaera sedula. J Ind Microbiol Biotechnol 43:1313–1321. https://doi.org/10.1007/s10295-016-1793-z
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (NSFC-21776208, NSFC-21621004 and NSFC-21776209).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Mao, Y., Wang, B. et al. Screening, expression, purification and characterization of CoA-transferases for lactoyl-CoA generation. J Ind Microbiol Biotechnol 46, 899–909 (2019). https://doi.org/10.1007/s10295-019-02174-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-019-02174-6