Abstract
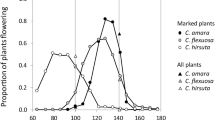
Time of flower anthesis in a day is thought to evolve in response to the time of pollinator activities. We studied blooming and withering time in natural populations of daylily (Hemerocallis fulva), nightlily (Hemerocallis citrina) and their hybrids, and also in an artificially obtained array of the F1 hybrids. Blooming time of H. fulva varied from 4:30 to 7:30 and H. citrina varied from 16:30 to 20:30. In a natural hybrid population, blooming time and withering time showed discontinuous bimodal distribution in spite that morphological traits of flowers showed continuous unimodal variation. Most F1 hybrids showed diurnal flowering. These findings indicate that only a few genes have strong phenotypic effect on the determination of flowering time in Hemerocallis, and suggest that the evolution from a H. fulva-like ancestor to H. citrina was not a continuous process by accumulation of minute mutations.





Similar content being viewed by others
References
Campbell DR (2004) Natural selection in Ipomopsis hybrid zones: implications for ecological speciation. New Phytol 161:83–90
Grant V (1992a) Floral isolation between ornithophilous and sphingophilous species of Ipomopsis and Aquilegia. Proc Natl Acad Sci USA 89:11828–11831
Grant V (1992b) Systematics and phylogeny of the Ipomopsis-aggregata group (Polemoniaceae)—traditional and molecular approaches. Syst Bot 17:683–691
Grant V (1993) Origin of floral isolation between ornithophilous and sphingophilous plant-species. Proc Natl Acad Sci USA 90:7729–7733
Hodges SA, Fulton M, Yang YJ, Whittall BJ (2004) Verne Grant and evolutionary studies of Aquilegia. New Phytol 161:113–120
Hotta M, Ito M, Okada I (1984) Anthesis of the genus Hemerocallis and its variation. Special mentions to nocturnal H. thunbergii of Tsushima & Hirado Islands, western Japan. Acta Phytotax Geobot 35:84–93 (in Japanese)
Hotta M, Ito M, Okada I (1985) Differentiation and species relationships of island population of Hemerocallis around Kyushu, Japan. In: Hara H (ed) Origin and evolution of diversity in plants and plant communities. Academia Scientific Book Inc., Tokyo, pp 18–30
Johnson SD, Edwards TJ, Carbutt C, Potgieter C (2002) Specialization for hawkmoth and long-proboscid fly pollination in Zaluzianskya section Nycterinia (Scrophulariaceae). Bot J Linn Soc 138:17–27
Kang SK, Chung MG (2000) High levels of allozyme variation within populations and low allozyme divergence within and among species of Hemerocallis (Liliaceae). Am J Bot 87:1634–1646
Kawano S (1961) On the natural hybrid population of Hemerocallis. Can J Bot 39:667–681
Kawano S, Noguchi J (1973) Biosystematic studies on the genus Hemerocallis (Liliaceae). I. Introgressive hybridization between H. citrina v. vespertina and H. fulva sensu lato. J Coll Lib Arts Toyama Univ 6:111–137
Matsuoka M, Hotta M (1966) Classification of Hemerocallis in Japan and its vicinity. Acta Phytotax Geobot 22:25–43 (in Japanese)
Nakao S, Yamashita K (1956) Variation in some plant populations. In: Komai T, Sakai K (eds) Syudan Idengaku. Baifukan, Tokyo, pp 243–259 (in Japanese)
Piechulla B (1989) Changes of the diurnal and circadian (endogenous) mRNA oscillations of the chlorophyll a/b binding protein in tomato leaves during altered day/night (light/night) regimes. Plant Mol Biol 12:317–327
Stout AB (1946) Types of anthesis in Hemerocallis and their heredity in F1 hybrids. Bull Torrey Bot Club 73:134–154
Tamura MN, Yamashita J, Fuse S, Haraguchi M (2004) Molecular phylogeny of monocotyledons inferred from combined analysis of plastid matK and rbcL gene sequences. J Pl Res 117:109–120
van der Pijl L (1961) Ecological aspects of flower evolution. II. Zoophilous flower class. Evolution 15:44–59
van Doorn WG, van Meeteren U (2003) Flower opening and closure: a review. J Exp Bot 54:1801–1812
Yanovsky MJ, Kay SA (2003) Living by the calendar: how plants know when to flower. Nat Rev Mol Cell Bio 4:265–275
Acknowledgements
We thank Eiichi Kasuya and Takashi Miyake for their critical comments on the manuscript, Yoh Iwasa, Hidenori Tachida, and Shiro Kobayashi for their valuable comments on the project, and Michikazu Hiramatsu for his kind help in the field and in the nursery of University Farm.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasegawa, M., Yahara, T., Yasumoto, A. et al. Bimodal distribution of flowering time in a natural hybrid population of daylily (Hemerocallis fulva) and nightlily (Hemerocallis citrina). J Plant Res 119, 63–68 (2006). https://doi.org/10.1007/s10265-005-0241-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-005-0241-3