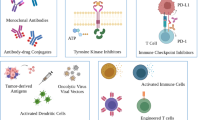
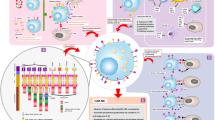
Abstract
Cancer remains a major health problem despite numerous new medical interventions that have been introduced in recent years. One of the major choices for cancer therapy is so-called adoptive cell therapy (ACT). ACT can be performed using both innate immune cells, including dendritic cells (DCs), natural killer (NK) cells, and γδ T cells and acquired immune T cells. It has become possible to utilize these cells in both their native and modified states in clinical studies. Because of considerable success in cancer treatment, ACT now plays a role in advanced therapy protocols. Genetic engineering of autologous and allogeneic immune cells (T lymphocytes, NK cells, macrophages, etc.) with chimeric antigen receptors (CAR) is a powerful new tool to target specific antigens on cancer cells. The Food and Drug Administration (FDA) in the US has approved certain CAR-T cells for hematologic malignancies and it is hoped that their use can be extended to incorporate a variety of cells, in particular NK cells. However, the ACT method has some limitations, such as the risk of rejection in allogeneic engrafts. Accordingly, numerous efforts are being made to eliminate or minimize this and other complications. In the present review, we have developed a guide to breast cancer (BC) therapy from conventional therapy, through to cell-based approaches, in particular novel technologies including CAR with emphasis on NK cells as a new and safer candidate in this field as well as the more recent aptamer technology, which can play a major role in BC immunotherapy.


Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- ACT:
-
Adoptive cell therapy
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- AE:
-
Adverse effect
- ApEn-NK:
-
Aptamer engineered-NK
- BC:
-
Breast cancer
- BiKE:
-
Bi-specific killer engager
- BiTE:
-
Bispecific T-cell engager
- CAR:
-
Chimeric antigen receptor
- CEA:
-
Carcinoembryonic antigen
- CIK:
-
Cytokine-induced killer
- CM:
-
Co-stimulatory molecule
- CRS:
-
Cytokine release syndrome
- CSC:
-
Cancer stem cells
- CTL:
-
Cytotoxic T-lymphocyte
- CTLA-4:
-
Cytotoxic T lymphocyte antigen-4
- DC:
-
Dendritic cell
- DCV:
-
DC-based vaccine
- EGFR:
-
Epidermal growth factor receptor
- EpCAM:
-
Epithelial cell adhesion molecule
- FDA:
-
Food and Drug Administration
- GD2:
-
Disialoganglioside
- GvHD:
-
Graft-versus-host disease
- HSPC:
-
Hematopoietic stem and progenitor cells
- HSV:
-
Herpes simplex virus
- HSV-tk:
-
HSV-1 thymidine kinase
- ICIs:
-
Immune checkpoint inhibitors
- IFN:
-
Interferon
- IL:
-
Interleukin
- iNKT:
-
Invariant natural killer T
- iPSC:
-
Induced pluripotent stem cell
- LAK:
-
Lymphokine-activated killer cells
- mAb:
-
Monoclonal antibody
- mBC:
-
Metastatic breast cancer
- MDSC:
-
Myeloid-derived suppressor cell
- MHC:
-
Major histocompatibility complex
- mTOR:
-
Mammalian target of rapamycin
- MUC:
-
Mucin
- NK:
-
Natural killer
- NKT:
-
Natural killer T
- OV:
-
Oncolytic viruses
- PBMC:
-
Peripheral blood mononuclear cell
- PD-1:
-
Programmed cell death protein 1
- pNK:
-
Primary NK
- ROR:
-
Receptor tyrosine kinase-like orphan receptor
- scFv:
-
Single-chain variable fragment
- TAA:
-
Tumor-associated antigen
- TCR-T:
-
T-cell receptor engineered T
- TGF-β:
-
Transforming growth factor beta
- TIL:
-
Tumor-infiltrating lymphocytes
- TME:
-
Tumor microenvironment
- TNBC:
-
Triple-negative breast cancer
- TRUCK:
-
T cells redirected for universal cytokine killing
- T-VEC:
-
Talimogene laherparepvec
- UCB:
-
Umbilical cord blood
- VEGF:
-
Vascular endothelial growth factor
- VV:
-
Vaccinia virus
References
Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. Asian Pac J Cancer Prev. 2019;20(7):2015–20.
Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–68.
Yousef AJA. Male breast cancer: epidemiology and risk factors. Semin Oncol. 2017;44(4):267–72.
Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–40.
Sharma GN, Dave R, Sanadya J, Sharma P, Sharma K. Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 2010;1(2):109–26.
Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clin Med Insights Pathol. 2015;8:23–31.
Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50(1):1–23.
Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23(5):489–502.
Moo T-A, Sanford R, Dang C, Morrow M. Overview of breast cancer therapy. PET clin. 2018;13(3):339–54.
Chew HK. Adjuvant therapy for breast cancer: who should get what? West J Med. 2001;174(4):284–7.
Verrill M. Chemotherapy for early-stage breast cancer: a brief history. Br J Cancer. 2009;101(1):S2–5.
Abderrahman B, Jordan VC. Improving long-term adjuvant anti-oestrogenic therapy for breast cancer. Clin Pharmacist. 2016. https://doi.org/10.1211/CP.2016.20201203.
Couzin-Frankel J. Cancer immunotherapy. Science. 2013;342:1432–3.
Provenzano E, Ulaner GA, Chin SF. Molecular classification of breast cancer. PET Clin. 2018;13(3):325–38.
Thomas R, Al-Khadairi G, Decock J. Immune checkpoint inhibitors in triple negative breast cancer treatment: promising future prospects. Front Oncol. 2020;10: 600573.
Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol. 2016;34(25):3069–103.
Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102(1–5):89–96.
Boér K. Fulvestrant in advanced breast cancer: evidence to date and place in therapy. Ther Adv Med Oncol. 2017;9(7):465–79.
Ji X, Lu Y, Tian H, Meng X, Wei M, Cho WC. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother. 2019;114: 108800.
García-Aranda M, Redondo M. Immunotherapy: a challenge of breast cancer treatment. Cancers. 2019;11(12):1822.
Hunter P. The fourth pillar: despite some setbacks in the clinic, immunotherapy has made notable progress toward becoming an additional therapeutic option against cancer. EMBO Rep. 2017;18(11):1889–92.
McCune JS. Rapid advances in immunotherapy to treat cancer. Clin Pharmacol Ther. 2018;103:540–4.
Marei HE, Althani A, Caceci T, et al. Recent perspective on CAR and Fcγ-CR T cell immunotherapy for cancers: Preclinical evidence versus clinical outcomes. Biochem pharmacol. 2019;166:335–46.
Kokate R. A systematic overview of cancer immunotherapy: an emerging therapy. Pharm Pharmacol Int J. 2017;5:31–5.
Pallerla S, Comeau J, Jois S. Cancer vaccines, treatment of the future: with emphasis on HER2-positive breast cancer. Int J Mol Sci. 2021;22(2):779.
Stoff-Khalili M, Dall P, Curiel D. Gene therapy for carcinoma of the breast. Cancer Gene Ther. 2006;13(7):633–47.
Sheikh-Hosseini M, Larijani B, Kakroodi ZG, et al. Gene therapy as an emerging therapeutic approach to breast cancer: new developments and challenges. Hum Gene Ther. 2021;32:1330–45.
Fisusi FA, Akala EO. Drug combinations in breast cancer therapy. Pharmaceutical nanotechnol. 2019;7(1):3–23.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72.
Sama CB, Dzekem B, Kehbila J, et al. Awareness of breast cancer and breast self-examination among female undergraduate students in a higher teachers training college in Cameroon. Pan Afr Med J. 2017;28(1):91.
Nde FP, Assob JCN, Kwenti TE, Njunda AL, Tainenbe TRG. Knowledge, attitude and practice of breast self-examination among female undergraduate students in the University of Buea. BMC Res Notes. 2015;8(1):43.
Qiu Y, Su M, Liu L, Tang Y, Pan Y, Sun J. Clinical application of cytokines in cancer immunotherapy. Drug Des Devel Ther. 2021;15:2269–88.
Bruera S, Leung CK. Immune-related adverse events with other cancer immunotherapies. In: Suarez-Almazor ME, Calabrese LH, editors. Rheumatic diseases and syndromes induced by cancer immunotherapy: a handbook for diagnosis and management. Cham: Springer; 2021. p. 255–69. https://doi.org/10.1007/978-3-030-56824-5_11.
Romee R, Leong JW, Fehniger TA. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica. 2014;2014: 205796.
Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Growth Factor Rev. 2006;17(5):325–37.
Kimmick G, Ratain MJ, Berry D, Woolf S, Norton L, Muss HB. Subcutaneously administered recombinant human interleukin-2 and interferon alfa-2a for advanced breast cancer: a phase II study of the Cancer and Leukemia Group B (CALGB 9041). Inves New Drugs. 2004;22(1):83–9.
Fallon JK, Vandeveer AJ, Schlom J, Greiner JW. Enhanced antitumor effects by combining an IL-12/anti-DNA fusion protein with avelumab, an anti-PD-L1 antibody. Oncotarget. 2017;8(13):20558–71.
Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. 2020;9(3):34.
Reslan L, Dalle S, Dumontet C. Understanding and circumventing resistance to anticancer monoclonal antibodies. MAbs. 2009;1(3):222–9.
Barzaman K, Moradi-Kalbolandi S, Hosseinzadeh A, Kazemi MH, Khorramdelazad H, Safari E, Farahmand L. Breast cancer immunotherapy: Current and novel approaches. Int Immunopharmacol. 2021;98: 107886.
Derakhshani A, Rezaei Z, Safarpour H, et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol. 2020;235(4):3142–56.
Lavaud P, Andre F. Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med. 2014;12(1):132.
Yu S, Liu Q, Han X, et al. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol. 2017;6(1):31.
Bernard-Marty C, Lebrun F, Awada A, Piccart MJ. Monoclonal antibody-based targeted therapy in breast cancer. Drugs. 2006;66(12):1577–91.
Mohit E, Hashemi A, Allahyari M. Breast cancer immunotherapy: monoclonal antibodies and peptide-based vaccines. Expert Rev Clin Immunol. 2014;10(7):927–61.
Naujokat C. Targeting human cancer stem cells with monoclonal antibodies. J Clin Cell Immunol S. 2012;5:007.
Cioffi M, Dorado J, Baeuerle PA, Heeschen C. EpCAM/CD3-bispecific t-cell engaging antibody MT110 eliminates primary human pancreatic cancer stem cells immunotherapy against pancreatic cancer stem cells. Clin Cancer Res. 2012;18(2):465–74.
Herrmann I, Baeuerle PA, Friedrich M, et al. Highly efficient elimination of colorectal tumor-initiating cells by an EpCAM/CD3-bispecific antibody engaging human T cells. PLoS ONE. 2010;5(10): e13474.
Dillon PM, Tushir-Singh J, Lum LG. Bispecific antibodies for the treatment of breast cancer. Expert Opin Biol Ther. 2022;22(8):1017–27.
Kebenko M, Goebeler ME, Wolf M, et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology. 2018;7(8): e1450710.
Zhou S, Liu M, Ren F, Meng X, Yu J. The landscape of bispecific T cell engager in cancer treatment. Biomark Res. 2021;9(1):38.
Wang Q, Chen Y, Park J, et al. Design and production of bispecific antibodies. Antibodies. 2019;8(3):43.
Dees S, Ganesan R, Singh S, Grewal IS. Bispecific antibodies for triple negative breast cancer. Trends Cancer. 2021;7(2):162–73.
Formenti SC, Lee P, Adams S, et al. Focal irradiation and systemic TGFβ blockade in metastatic breast cancerradiation and TGFβ blockade in metastatic breast cancer. Clin Cancer Res. 2018;24(11):2493–504.
Parihar R, Nadella P, Lewis A, et al. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon γ production in a subset of patients. Clin Cancer Res. 2004;10(15):5027–37.
Mani A, Roda J, Young D, et al. A phase II trial of trastuzumab in combination with low-dose interleukin-2 (IL-2) in patients (PTS) with metastatic breast cancer (MBC) who have previously failed trastuzumab. Breast Cancer Res Treat. 2009;117:83–9.
Sanz L, Álvarez-Vallina L. Engineered mRNA and the rise of next-generation antibodies. Antibodies. 2021;10(4):37.
Kakimi K, Karasaki T, Matsushita H, Sugie T. Advances in personalized cancer immunotherapy. Breast Cancer. 2017;24(1):16–24.
González-Navajas JM, Fan DD, Yang S, et al. The impact of Tregs on the anticancer immunity and the efficacy of immune checkpoint inhibitor therapies. Front Immunol. 2021;12: 625783.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
Emens LA. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res. 2018;24(3):511–20.
Chauvin J-M, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2): e000957.
Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004.
Stovgaard ES, Nielsen D, Hogdall E, Balslev E. Triple negative breast cancer–prognostic role of immune-related factors: a systematic review. Acta Oncol. 2018;57(1):74–82.
Kagihara JA, Andress M, Diamond JR. Nab-paclitaxel and atezolizumab for the treatment of PD-L1-positive, metastatic triple-negative breast cancer: review and future directions. Expert Rev Precis Med Drug Dev. 2020;5(2):59–65.
Vonderheide RH, Domchek SM, Clark AS. Immunotherapy for breast cancer: what are we missing? Clin Cancer Res. 2017;23:2640–6.
Majidpoor J, Mortezaee K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin Immunol. 2021;226:108707.
Draganov D, Han Z, Rana A, Bennett N, Irvine DJ, Lee PP. Ivermectin converts cold tumors hot and synergizes with immune checkpoint blockade for treatment of breast cancer. NPJ Breast Cancer. 2021;7(1):22.
Kim K, Skora AD, Li Z, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111(32):11774–9.
Loi S, Dushyanthen S, Beavis PA, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res. 2016;22(6):1499–509.
Kennedy LB, Salama AK. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104.
Chhabra N, Kennedy J. A review of cancer immunotherapy toxicity: immune checkpoint inhibitors. J Med Toxicol. 2021;17:411–24.
Naing A, Thistlethwaite F, De Vries EG, et al. CX-072 (pacmilimab), a Probody® PD-L1 inhibitor, in advanced or recurrent solid tumors (PROCLAIM-CX-072): an open-label dose-finding and first-in-human study. J Immunother Cancer. 2021;9(7): e002447.
Sanborn RE, Hamid O, de Vries EG, et al. CX-072 (pacmilimab), a Probody PD-L1 inhibitor, in combination with ipilimumab in patients with advanced solid tumors (PROCLAIM-CX-072): a first-in-human, dose-finding study. J Immunother Cancer. 2021;9(7): e002446.
Zhu SY, Yu KD. Breast cancer vaccines: disappointing or promising? Front Immunol. 2022;13: 828386.
Behravan J, Razazan A, Behravan G. Towards breast cancer vaccines, progress and challenges. Curr Drug Discov Technol. 2019;16(3):251–8.
Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4(1):7.
Mittendorf EA, Lu B, Melisko M, et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019;25(14):4248–54.
Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106(3):271–4.
Arab A, Yazdian-Robati R, Behravan J. HER2-Positive breast cancer immunotherapy: a focus on vaccine development. Arch Immunol Ther Exp. 2020;68(1):2.
Fuentes-Antras J, Guevara-Hoyer K, Baliu-Piqué M, et al. Adoptive cell therapy in breast cancer: a current perspective of next-generation medicine. Front Oncol. 2020;10: 605633.
Safety Study Of Chemotherapy Combined With Dendritic Cell Vaccine to Treat Breast Cancer. https://clinicaltrials.gov/ct2/show/study/NCT02018458?term=NCT02018458&draw=2&rank=1
O’Shaughnessy J, Roberts LK, Smith JL, et al. Safety and initial clinical efficacy of a dendritic cell (DC) vaccine in locally advanced, triple-negative breast cancer (TNBC) patients (pts). J Clin Oncol. 2016;34(15suppl):1086.
Sutherland SI, Ju X, Horvath L, Clark GJ. Moving on from Sipuleucel-T: new dendritic cell vaccine strategies for prostate cancer. Front Immunol. 2021;12: 641307.
Mair F, Liechti T. Comprehensive phenotyping of human dendritic cells and monocytes. Cytometry A. 2021;99(3):231–42.
Bouzid R, Peppelenbosch M, Buschow SI. Opportunities for conventional and in situ cancer vaccine strategies and combination with immunotherapy for gastrointestinal cancers, a review. Cancers. 2020;12(5):1121.
Lopes A, Vandermeulen G, Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res. 2019;38(1):146.
Li S, Wu J, Li X, Chen J, Wang C. Biomaterial-enhanced cancer vaccines. Mater Des. 2022;218: 110720.
Suryawanshi YR, Zhang T, Essani K. Oncolytic viruses: emerging options for the treatment of breast cancer. Med Oncol. 2017;34(3):43.
Cejalvo JM, Falato C, Villanueva L, et al. Oncolytic viruses: a new immunotherapeutic approach for breast cancer treatment? Cancer Treat Rev. 2022;106: 102392.
Javanbakht M, Tahmasebzadeh S, Cegolon L, et al. Oncolytic Viruses: a novel treatment strategy for breast cancer. Genes Dis. 2021. https://doi.org/10.1016/j.gendis.2021.11.011.
Zhu W, Wei L, Zhang H, Chen J, Qin X. Oncolytic adenovirus armed with IL-24 inhibits the growth of breast cancer in vitro and in vivo. J Exp Clin Cancer Res. 2012;31(1):51.
Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–47.
Kai M, Marx AN, Liu DD, et al. A phase II study of talimogene laherparepvec for patients with inoperable locoregional recurrence of breast cancer. Sci Rep. 2021;11(1):22242.
Talimogene Laherparepvec in Combination With Neoadjuvant Chemotherapy in Triple Negative Breast Cancer. https://clinicaltrials.gov/ct2/show/NCT02779855?term=NCT02779855&draw=2&rank=1
Luo C, Wang P, He S, Zhu J, Shi Y, Wang J. Progress and prospect of immunotherapy for triple-negative breast cancer. Front Oncol. 2022;12: 919072.
Immunization Strategy With Intra-tumoral Injections of Pexa-Vec With Ipilimumab in Metastatic / Advanced Solid Tumors. (ISI-JX). https://clinicaltrials.gov/ct2/show/NCT02977156?term=NCT02977156&draw=2&rank=1
SBRT and Oncolytic Virus Therapy Before Pembrolizumab for Metastatic TNBC and NSCLC (STOMP). https://clinicaltrials.gov/ct2/show/NCT03004183?term=NCT03004183&draw=2&rank=1
Hossain JA, Riecken K, Miletic H, Fehse B. Cancer suicide gene therapy with TK. 007. In: Düzgüneş N, editor. Suicide gene therapy methods in molecular biology, vol. 1895. New York: Humana Press; 2019. p. 11–26.
Zhao Q, Jiang Y, Xiang S, et al. Engineered TCR-T cell immunotherapy in anticancer precision medicine: pros and cons. Front Immunol. 2021;12: 658753.
Rohaan MW, Wilgenhof S, Haanen JB. Adoptive cellular therapies: the current landscape. Virchows Arch. 2019;474(4):449–61.
Kang S, Gao X, Zhang L, Yang E, Li Y, Yu L. The advances and challenges of NK cell-based cancer immunotherapy. Curr Oncol. 2021;28(2):1077–93.
Besser MJ, Shapira-Frommer R, Itzhaki O, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19(17):4792–800.
Nelson MA, Ngamcherdtrakul W, Luoh S-W, Yantasee W. Prognostic and therapeutic role of tumor-infiltrating lymphocyte subtypes in breast cancer. Cancer Metastasis Rev. 2021;40(2):519–36.
Li D, Li X, Zhou WL, et al. Genetically engineered T cells for cancer immunotherapy. Signal Transduct Target Ther. 2019;4(1):35.
Autologous Tumor Infiltrating Lymphocytes in Patients With Pretreated Metastatic Triple Negative Breast Cancer. https://clinicaltrials.gov/ct2/show/NCT04111510?term=NCT04111510&draw=2&rank=1
Immunotherapy Using Tumor Infiltrating Lymphocytes for Patients With Metastatic Cancer. https://clinicaltrials.gov/ct2/show/NCT01174121?term=NCT01174121&draw=2&rank=1
Engineered TILs/CAR-TILs to Treat Advanced Solid Tumors. Available from: https://clinicaltrials.gov/ct2/show/NCT04842812?term=NCT04842812&draw=2&rank=1
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–5.
Kumar A, Watkins R, Vilgelm AE. Cell therapy with TILs: training and taming T cells to fight cancer. Front Immunol. 2021;12: 690499.
Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–6.
Wang L, Liu J. Engineered drug-loaded cells and cell derivatives as a delivery platform for cancer immunotherapy. Biomater Sci. 2021;6:1104–16.
Morgan MA, Büning H, Sauer M, Schambach A. Use of cell and genome modification technologies to generate improved “off-the-shelf” CAR T and CAR NK Cells. Front Immunol. 2020;11:1965.
Fousek K, Watanabe J, Joseph SK, et al. CAR T-cells that target acute B-lineage leukemia irrespective of CD19 expression. Leukemia. 2021;35(1):75–89.
Voynova E, Kovalovsky D. From hematopoietic stem cell transplantation to chimeric antigen receptor therapy: advances, limitations and future perspectives. Cells. 2021;10(11):2845.
Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine. 2020;59: 102975.
Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145–54.
Basar R, Daher M, Rezvani K. Next-generation cell therapies: the emerging role of CAR-NK cells. Hematol Am Soc Hematol Educ Program. 2020;2020(1):570–8.
Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: clinical transformation and future prospects. Cancer Lett. 2020;472:175–80.
Tokarew N, Ogonek J, Endres S, von Bergwelt-Baildon M, Kobold S. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer. 2019;120(1):26–37.
Elahi R, Khosh E, Tahmasebi S, Esmaeilzadeh A. Immune cell hacking: challenges and clinical approaches to create smarter generations of chimeric antigen receptor T cells. Front Immunol. 2018;9:1717.
Knochelmann HM, Smith AS, Dwyer CJ, Wyatt MM, Mehrotra S, Paulos CM. CAR T cells in solid tumors: blueprints for building effective therapies. Front Immunol. 2018;9:1740.
Garrido F, Aptsiauri N, Doorduijn EM, Lora AMG, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51.
Helmy KY, Patel SA, Nahas GR, Rameshwar P. Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv. 2013;4(10):1307–20.
Xu Q, Harto H, Berahovich R, et al. Generation of CAR-T cells for cancer immunotherapy. Methods Mol Biol. 2019;1884:349–60.
Graham C, Jozwik A, Pepper A, Benjamin R. Allogeneic CAR-T cells: more than ease of access? Cells. 2018;7(10):155.
Han D, Xu Z, Zhuang Y, Ye Z, Qian Q. Current progress in CAR-T cell therapy for hematological malignancies. J Cancer. 2021;12(2):326–34.
Liu D. CAR-T “the living drugs”, immune checkpoint inhibitors, and precision medicine: a new era of cancer therapy. J Hematol Oncol. 2019;12:113.
Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257(1):56–71.
Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52.
Zhou R, Yazdanifar M, Roy LD, et al. CAR T cells targeting the tumor MUC1 glycoprotein reduce triple-negative breast cancer growth. Front Immunol. 2019;10:1149.
Wei J, Sun H, Zhang A, et al. A novel AXL chimeric antigen receptor endows T cells with anti-tumor effects against triple negative breast cancers. Cell Immunol. 2018;331:49–58.
Xia L, Zheng ZZ, Liu JY, et al. EGFR-targeted CAR-T cells are potent and specific in suppressing triple-negative breast cancer both in vitro and in vivo. Clin Transl Immunol. 2020;9(5): e1135.
Seitz CM, Schroeder S, Knopf P, et al. GD2-targeted chimeric antigen receptor T cells prevent metastasis formation by elimination of breast cancer stem-like cells. Oncoimmunology. 2020;9(1):1683345.
Wei H, Wang Z, Kuang Y, et al. Intercellular adhesion molecule-1 as target for CAR-T-cell therapy of triple-negative breast cancer. Front Immunol. 2020;11: 573823.
Wallstabe L, Göttlich C, Nelke LC, et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI insight. 2019;4(18):e126345.
Song DG, Ye Q, Poussin M, Chacon JA, Figini M, Powell DJ. Effective adoptive immunotherapy of triple-negative breast cancer by folate receptor-alpha redirected CAR T cells is influenced by surface antigen expression level. J Hematol Oncol. 2016;9(1):1–12.
Han Y, Xie W, Song DG, Powell DJ. Control of triple-negative breast cancer using ex vivo self-enriched, costimulated NKG2D CAR T cells. J Hematol Oncol. 2018;11(1):1–13.
Dees S, Ganesan R, Singh S, Grewal IS. Emerging CAR-T cell therapy for the treatment of triple-negative breast cancer. Mol Cancer Ther. 2020;19(12):2409–21.
Bajgain P, Tawinwung S, D’Elia L, et al. CAR T cell therapy for breast cancer: harnessing the tumor milieu to drive T cell activation. J Immunother Cancer. 2018;6(1):34.
Zhao Z, Li Y, Liu W, Li X. Engineered IL-7 receptor enhances the therapeutic effect of AXL-CAR-T cells on triple-negative breast cancer. BioMed Res Int. 2020;2020:4795171.
Szöőr Á, Tóth G, Zsebik B, et al. Trastuzumab derived HER2-specific CARs for the treatment of trastuzumab-resistant breast cancer: CAR T cells penetrate and eradicate tumors that are not accessible to antibodies. Cancer lett. 2020;484:1–8.
Zhou F, Krishnamurthy J, Wei Y, et al. Chimeric antigen receptor T cells targeting HERV-K inhibit breast cancer and its metastasis through downregulation of Ras. Oncoimmunology. 2015;4(11): e1047582.
Wilkie S, van Schalkwyk MC, Hobbs S, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clinical Immunol. 2012;32(5):1059–70.
Rozenbaum M, Meir A, Aharony Y, et al. Gamma-delta CAR-T cells show CAR-directed and independent activity against leukemia. Front Immunol. 2020;11:1347.
Haplo / Allogeneic NKG2DL-targeting Chimeric Antigen Receptor-grafted γδ T Cells for Relapsed or Refractory Solid Tumour. https://clinicaltrials.gov/ct2/show/NCT04107142?term=NCT04107142&draw=2&rank=1
Choi BD, Yu X, Castano AP, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37(9):1049–58.
Wang J, Zhou P. New approaches in CAR-T cell immunotherapy for breast cancer. In: Song E, Hu H, editors. Translational research in breast cancer. Singapore: Springer; 2017. p. 371–81.
Ramello MC, Haura EB, Abate-Daga D. CAR-T cells and combination therapies: What’s next in the immunotherapy revolution? Pharmacol research. 2018;129:194–203.
Li H, Yuan W, Bin S, et al. Overcome trastuzumab resistance of breast cancer using anti-HER2 chimeric antigen receptor T cells and PD1 blockade. Am J Cancer Res. 2020;10(2):688–703.
Sun R, Luo H, Su J, et al. Olaparib suppresses MDSC recruitment via SDF1α/CXCR4 axis to improve the anti-tumor efficacy of CAR-T cells on breast cancer in mice. Mol Ther. 2021;29(1):60–74.
Caratelli S, Arriga R, Sconocchia T, et al. In vitro elimination of epidermal growth factor receptor-overexpressing cancer cells by CD32A-chimeric receptor T cells in combination with cetuximab or panitumumab. Int J Cancer. 2020;146(1):236–47.
Stüber T, Monjezi R, Wallstabe L, et al. Inhibition of TGF-β-receptor signaling augments the antitumor function of ROR1-specific CAR T-cells against triple-negative breast cancer. J Immunother Cancer. 2020;8(1): e000676.
Li Y, Xiao F, Zhang A, et al. Oncolytic adenovirus targeting TGF-β enhances anti-tumor responses of mesothelin-targeted chimeric antigen receptor T cell therapy against breast cancer. Cell immunol. 2020;348: 104041.
Xu N, Palmer DC, Robeson AC, et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J Exp Med. 2020;218(2): e20200844.
Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25(8):1769–81.
Li C, Mei H, Hu Y. Applications and explorations of CRISPR/Cas9 in CAR T-cell therapy. Brief Funct Genomics. 2020;19(3):175–82.
Lee DA. Cellular therapy: adoptive immunotherapy with expanded natural killer cells. Immunol rev. 2019;290(1):85–99.
Davis ZB, Vallera DA, Miller JS, Felices M. Natural killer cells unleashed: Checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin Immunol. 2017;31:64–75.
Gabrielli S, Ortolani C, Del Zotto G, et al. The memories of NK cells: innate-adaptive immune intrinsic crosstalk. J Immunol Res. 2016;2016:1376595.
Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230–52.
Suck G, Odendahl M, Nowakowska P, et al. NK-92: an ‘off-the-shelf therapeutic’for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016;65(4):485–92.
Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy–advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7:91.
Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8(4):652–8.
Ferrari de Andrade L, Kumar S, et al. Inhibition of MICA and MICB shedding elicits NK-cell-mediated immunity against tumors resistant to cytotoxic T cells. Cancer Immunol Res. 2020;8(6):769–80.
Valipour B, Velaei K, Abedelahi A, Karimipour M, Darabi M, Charoudeh HN. NK cells: an attractive candidate for cancer therapy. J Cell Physiol. 2019;234(11):19352–65.
Zhang C, Liu Y. Targeting NK cell checkpoint receptors or molecules for cancer immunotherapy. Front Immunol. 2020;11:1179.
Lachota M, Vincenti M, Winiarska M, Boye K, Zagożdżon R, Malmberg KJ. Prospects for NK cell therapy of sarcoma. Cancers. 2020;12(12):3719.
Qian X, Wang X, Jin H. Cell transfer therapy for cancer: past, present, and future. J Immunol Res. 2014;2014: 525913.
Tay SS, Carol H, Biro M. TriKEs and BiKEs join CARs on the cancer immunotherapy highway. Hum Vaccin Immunother. 2016;12(11):2790–6.
Tabata R, Chi S, Yuda J, Minami Y. Emerging immunotherapy for acute myeloid leukemia. Int J Mol Sci. 2021;22(4):1944.
Cichocki F, Bjordahl R, Gaidarova S, et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci Transl Med. 2020;12(568):5618.
FT500 as Monotherapy and in Combination With Immune Checkpoint Inhibitors in Subjects With Advanced Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT03841110?term=NCT03841110&draw=2&rank=1
FT536 Monotherapy and in Combination With Monoclonal Antibodies in Advanced Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT05395052?term=NCT05395052&draw=1&rank=1
Chen X, Han J, Chu J, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. 2016;7(19):27764.
Liu Y, Zhou Y, Huang KH, et al. Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor. Cell Prolif. 2020;53(8): e12858.
Hu Z. Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci Rep. 2020;10(1):2815.
CAR-pNK Cell Immunotherapy in MUC1 Positive Relapsed or Refractory Solid Tumor. https://clinicaltrials.gov/ct2/show/NCT02839954?term=NCT02839954&draw=2&rank=1
Matosevic S. Viral and nonviral engineering of natural killer cells as emerging adoptive cancer immunotherapies. J Immunol Res. 2018;2018:4054815.
Simonetta F, Alvarez M, Negrin RS. Natural killer cells in graft-versus-host-disease after allogeneic hematopoietic cell transplantation. Front Immunol. 2017;8:465.
Motohashi S. The role of regenerative invariant NKT cells in cancer immunotherapy for head and neck cancer. Per Med Universe. 2022;11:14–9.
Mazinani M, Rahbarizadeh F. New cell sources for CAR-based immunotherapy. Biomark Res. 2023;11(1):49.
Liu X, Li L, Si F, et al. NK and NKT cells have distinct properties and functions in cancer. Oncogene. 2021;40(27):4521–37.
Hadiloo K, Tahmasebi S, Esmaeilzadeh A. CAR-NKT cell therapy: a new promising paradigm of cancer immunotherapy. Cancer Cell Int. 2023;23(1):86.
Gebremeskel S, Nelson A, Walker B, et al. Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis. J Immunother Cancer. 2021;9(3): e002096.
Heczey A, Xu X, Courtney AN, et al. Anti-GD2 CAR-NKT cells in relapsed or refractory neuroblastoma: updated phase 1 trial interim results. Nat Med. 2023;29(6):1379–88.
Zhang Y, Schmidt-Wolf IG. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J Cell Physiol. 2020;235(12):9291–303.
Guo Y, Han W. Cytokine-induced killer (CIK) cells: from basic research to clinical translation. Chin J Cancer. 2015;34(3):99–107.
Hombach AA, Rappl G, Abken H. Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28–OX40 “super-stimulation.” Mol Ther. 2013;21(12):2268–77.
Wongkajornsilp A, Htwe KSS, Sawatpiboon N, Duangsa-ard S, Kasetsinsombat K. The induction of iNKT cells and CIK cells toward anti-tumor phenotypes. Cancer Res. 2019;79(13 Supplement):4141.
Sharma A, Schmidt-Wolf IG. 30 years of CIK cell therapy: recapitulating the key breakthroughs and future perspective. J Exp Clin Cancer Res. 2021;40(1):388.
Duan H, Ji N, Zhao L, Shi Q, Qi Y, Qi Q. Cytokine-induced killer cells (CIK) from healthy donors for treatment of advanced breast cancer. Int J Clin Exp Med. 2018;11(12):13436–41.
Li M, Wang Y, Wei F, et al. Efficiency of cytokine-induced killer cells in combination with chemotherapy for triple-negative breast cancer. J Breast Cancer. 2018;21(2):150–7.
Pan K, Guan X-X, Li Y-Q, et al. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res. 2014;20(11):3003–11.
Hu J, Hu J, Liu X, Hu C, Li M, Han W. Effect and safety of cytokine-induced killer (CIK) cell immunotherapy in patients with breast cancer: a meta-analysis. Medicine. 2017;96(42): e8310.
A Study of Combinations of D-CIK Immunotherapy And Anti-PD-1 In Refractory Solid Tumors. https://classic.clinicaltrials.gov/ct2/show/NCT02886897?term=cytokine-induced+killer&cond=Breast+Cancer&phase=04&draw=2&rank=1
Merker M, Wagner J, Kreyenberg H, et al. ERBB2-CAR-engineered cytokine-induced killer cells exhibit both CAR-mediated and innate immunity against high-risk rhabdomyosarcoma. Front Immunol. 2020;11: 581468.
Magnani CF, Gaipa G, Lussana F, et al. Sleeping beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J Clin Invest. 2020;130(11):6021–33.
Ren X, Ma W, Lu H, et al. Modification of cytokine-induced killer cells with chimeric antigen receptors (CARs) enhances antitumor immunity to epidermal growth factor receptor (EGFR)-positive malignancies. Cancer Immunol Immunother. 2015;64:1517–29.
Mehta AK, Kadel S, Townsend MG, Oliwa M, Guerriero JL. Macrophage biology and mechanisms of immune suppression in breast cancer. Front Immunol. 2021;12: 643771.
CAR-macrophages for the Treatment of HER2 Overexpressing Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT04660929?term=NCT04660929&draw=2&rank=1
Cohort Study to Determine the Antitumor Activity of New CAR-macrophages in Breast Cancer Patients' Derived Organoids (CARMA). https://clinicaltrials.gov/ct2/show/NCT05007379?term=NCT05007379&draw=2&rank=1
Li WM, Zhou LL, Zheng M, Fang J. Selection of metastatic breast cancer cell-specific aptamers for the capture of CTCs with a metastatic phenotype by cell-SELEX. Mol Ther Nucleic Acids. 2018;12:707–17.
Han J, Gao L, Wang J, Wang J. Application and development of aptamer in cancer: from clinical diagnosis to cancer therapy. J Cancer. 2020;11(23):6902–15.
Zhou G, Wilson G, Hebbard L, et al. Aptamers: a promising chemical antibody for cancer therapy. Oncotarget. 2016;7(12):13446–63.
Liu M, Yu X, Chen Z, et al. Aptamer selection and applications for breast cancer diagnostics and therapy. J Nanobiotechnol. 2017;15(1):81.
Yang S, Li H, Xu L, et al. Oligonucleotide aptamer-mediated precision therapy of hematological malignancies. Mol Ther Nucleic Acids. 2018;13:164–75.
Kim DM, Kim M, Park HB, Kim KS, Kim DE. Anti-MUC1/CD44 dual-aptamer-conjugated liposomes for cotargeting breast cancer cells and cancer stem cells. ACS Appl Bio Mater. 2019;2(10):4622–33.
Camorani S, Passariello M, Agnello L, et al. Aptamer targeted therapy potentiates immune checkpoint blockade in triple-negative breast cancer. J Exp Clin Cancer Res. 2020;39(1):180.
Yang S, Wen J, Li H, et al. Aptamer-engineered natural killer cells for cell-specific adaptive immunotherapy. Small. 2019;15(22): e1900903.
Zhang D, Zheng Y, Lin Z, et al. Equipping natural killer cells with specific targeting and checkpoint blocking aptamers for enhanced adoptive immunotherapy in solid tumors. Angew Chem Int Ed Engl. 2020;59(29):12022–8.
Chen Z, Zeng Z, Wan Q, Liu X, Qi J, Zu Y. Targeted immunotherapy of triple-negative breast cancer by aptamer-engineered NK cells. Biomaterials. 2022;280: 121259.
Funding
The authors did not receive any funds from any organization for the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Writing-original draft preparation, SK; conceptualization, EV and RJ-S; review and editing, RJ-S, EV, and JRW; English language editing, JRW; supervision, EV and RJ-S; and illustration, SK. All authors read the last version of manuscript and approved it for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest related to this work.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keshavarz, S., Wall, J.R., Keshavarz, S. et al. Breast cancer immunotherapy: a comprehensive review. Clin Exp Med 23, 4431–4447 (2023). https://doi.org/10.1007/s10238-023-01177-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01177-z