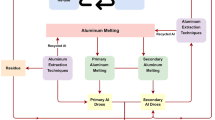
Abstract
Herein, fluorine fixation, nitrogen removal, and the alkaline solution recovery of alumina were studied using calcium-based high-temperature soda roasting. In addition, the effects of roasting conditions, dissolution conditions, and additives (carbon alkali and calcium salt) on fluorine fixation and alumina recovery were studied. The results show that when the roasting temperature was 1100 ℃ and the roasting time was 2 h, the dissolution temperature was 80 °C, while the dissolution time was 20 min. The ratio of soda to dross was 1.0, and 30 wt% CaCO3 had been added as a fluorine fixing agent. The Al extraction rate from aluminum dross (AD) reached 86.21%, the denitrification rate reached 99.54%, the concentration of soluble fluoride ion was reduced to 0.111 g·L−1, and the fluoride fixation rate was 75.04 wt%. CaF2 is the main component of the leaching residue, which can be added as an insulation material for electrolysis. Alkaline oxidation roasting and calcium-based fluoride fixation are effective methods to separate aluminum, fluoride, and nitrogen. This study provides a new method for the safe and efficient utilization of AD.











Similar content being viewed by others
References
Zhu X, Jin Q (2021) Comparison of three emerging dross recovery processes in China’s aluminum industry from the perspective of life cycle assessment. ACS Sustain Chem Eng 9:6776–6787. https://doi.org/10.1021/acssuschemeng.1c00960
Meshram A, Singh KK (2018) Recovery of valuable products from hazardous aluminum dross: a review. Resour Conserv Recycl 130:95–108. https://doi.org/10.1016/j.resconrec.2017.11.026
Mahinroosta M, Allahverdi A (2018) Hazardous aluminum dross characterization and recycling strategies: a critical review. J Environ Manage 223:452–468. https://doi.org/10.1016/j.jenvman.2018.06.068
Zuo Z, Lv H, Li R et al (2021) A new approach to recover the valuable elements in black aluminum dross. Resour Conserv Recycl. https://doi.org/10.1016/j.resconrec.2021.105768
Li P, Guo M, Zhang M et al (2012) Leaching process investigation of secondary aluminum dross: the effect of CO2 on leaching process of salt cake from aluminum remelting process. Metall Mater Trans B Process Metall Mater Process Sci 43:1220–1230. https://doi.org/10.1007/s11663-012-9678-7
Mahinroosta M, Allahverdi A (2018) A promising green process for synthesis of high purity activated-alumina nanopowder from secondary aluminum dross. J Clean Prod 179:93–102. https://doi.org/10.1016/j.jclepro.2018.01.079
David E, Kopac J (2013) Aluminum recovery as a product with high added value using aluminum hazardous waste. J Hazard Mater 261:316–324. https://doi.org/10.1016/j.jhazmat.2013.07.042
Wan B, Li W, Sun W et al (2020) Synthesis of cryolite (Na3AlF6) from secondary aluminum dross generated in the aluminum recycling process. Materials (Basel). https://doi.org/10.3390/ma13173871
Satish Reddy M, Neeraja D (2018) Aluminum residue waste for possible utilisation as a material: a review. Sadhana - Acad Proc Eng Sci 43:1–8. https://doi.org/10.1007/s12046-018-0866-2
Nguyen TTN, Song SJ, Lee MS (2020) Development of a hydrometallurgical process for the recovery of pure alumina from black dross and synthesis of magnesium spinel. J Mater Res Technol 9:2568–2577. https://doi.org/10.1016/j.jmrt.2019.12.087
Zhu W, Wu K, Zhang S et al (2021) Zero-waste progress for the synthesis of high-purity β-Sialon ceramics from secondary aluminum dross. Adv Eng Mater 23:1–9. https://doi.org/10.1002/adem.202001298
Ramaswamy P, Gomes SA, Ravichander NP (2019) Utilization of aluminum dross: refractories from industrial waste. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/577/1/012101
López FA, Martín MI, Alguacil FJ et al (2019) Synthesis of calcium aluminates from non-saline aluminum dross. Materials (Basel) 12:1–12. https://doi.org/10.3390/ma12111837
Hsieh KC, Ueng TH, Chen CC (2013) A study of stabilization and recycling for aluminum dross. Appl Mech Mater 275–277:2237–2240. https://doi.org/10.4028/www.scientific.net/AMM.275-277.2237
Beheshti R, Moosberg-Bustnes J, Akhtar S, Aune RE (2017) Black dross processing: utilization of black dross in the production of a ladle fluxing agent. J Sustain Metall 3:265–273. https://doi.org/10.1007/s40831-016-0076-2
Zhang Y, Guohui Z, Hanyu Z et al (2019) Feasibility of aluminum recovery and MgAl2O4 spinel synthesis from secondary aluminum dross. Int J Miner Metall Mater 26:309–318. https://doi.org/10.1007/s12613-019-1739-3
Li P, Zhang M, Wang Z, Seetharaman S (2015) BF slag resistance of β-Si3Al3O3N5 material derived from al salt cake. J Eur Ceram Soc 35:1307–1315. https://doi.org/10.1016/j.jeurceramsoc.2014.11.002
Wanghui J, Zhongqing Y, Tong Y et al (2021) Removal of AlN from secondary aluminum dross by pyrometallurgical treatment. J Cent South Univ 28:386–397. https://doi.org/10.1007/s11771-021-4610-4
Lv H, Zhao H, Zuo Z et al (2020) A thermodynamic and kinetic study of catalyzed hydrolysis of aluminum nitride in secondary aluminum dross. J Mater Res Technol 9:9735–9745. https://doi.org/10.1016/j.jmrt.2020.06.051
Li Q, Yang Q, Zhang G, Shi Q (2018) Investigations on the hydrolysis behavior of AlN in the leaching process of secondary aluminum dross. Hydrometallurgy 182:121–127. https://doi.org/10.1016/j.hydromet.2018.10.015
Shen H, Liu B, Ekberg C, Zhang S (2021) Harmless disposal and resource utilization for secondary aluminum dross: a review. Sci Total Environ 760:143968. https://doi.org/10.1016/j.scitotenv.2020.143968
LiuyangXiang HN, Shenyi X et al (2020) Decrease of Material Burden in a Novel Alkali-Saving Reduction Treatment Process of Nickel Slag Based on NaOH Roasting. Jom 72:2686–2696. https://doi.org/10.1007/s11837-019-03914-w
Li D, Jiang X, Wang S et al (2019) Research on the alkali-digestion properties of alumina and silicon dioxide during phase transformation roasting process. Fuel Process Technol 191:223–231. https://doi.org/10.1016/j.fuproc.2019.04.013
Li X, Ou Y, Li C et al (2019) Preparation of alumina from aluminum Ash by sintering with sodium hydroxide. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/233/4/042027
Guo H, Wang J, Zhang X et al (2018) Study on the extraction of aluminum from aluminum dross using alkali roasting and subsequent synthesis of mesoporous γ-alumina. Metall Mater Trans B Process Metall Mater Process Sci 49:2906–2916. https://doi.org/10.1007/s11663-018-1341-5
He L, Shi L, Huang Q et al (2021) Extraction of alumina from aluminum dross by a non-hazardous alkaline sintering process: dissolution kinetics of alumina and silica from calcined materials. Sci Total Environ 777:146123. https://doi.org/10.1016/j.scitotenv.2021.146123
Dash B, Das BR, Tripathy BC et al (2008) Acid dissolution of alumina from waste aluminium dross. Hydrometallurgy 92:48–53. https://doi.org/10.1016/j.hydromet.2008.01.006
Sarker MSR, Alam MZ, Qadir MR et al (2015) Extraction and characterization of alumina nanopowders from aluminum dross by acid dissolution process. Int J Miner Metall Mater 22:429–436. https://doi.org/10.1007/s12613-015-1090-2
Türk M, Altıner M, Top S et al (2020) Production of alpha-alumina from black aluminum dross using NaOH leaching followed by calcination. Jom 72:3358–3366. https://doi.org/10.1007/s11837-020-04281-7
Feng H, Zhang G, Yang Q et al (2020) The investigation of optimizing leaching efficiency of al in secondary aluminum dross via pretreatment operations. Processes 8:1–13. https://doi.org/10.3390/pr8101269
Bruckard WJ, Woodcock JT (2007) Characterisation and treatment of Australian salt cakes by aqueous leaching. Miner Eng 20:1376–1390. https://doi.org/10.1016/j.mineng.2007.08.020
Tsakiridis PE, Oustadakis P, Agatzini-Leonardou S (2013) Aluminium recovery during black dross hydrothermal treatment. J Environ Chem Eng 1:23–32. https://doi.org/10.1016/j.jece.2013.03.004
Gao Q, Guo Q, Li Y et al (2021) Innovative technology for defluorination of secondary aluminum dross by alkali leaching. Miner Eng 172:107134. https://doi.org/10.1016/j.mineng.2021.107134
Cornell RM, Schwertmann U (2003) Thermodynamics of the Fe-O 2 -H 2 O system. Iron Oxides. https://doi.org/10.1002/3527602097.ch8
Tripathy AK, Mahalik S, Sarangi CK et al (2019) A pyro-hydrometallurgical process for the recovery of alumina from waste aluminium dross. Miner Eng 137:181–186. https://doi.org/10.1016/j.mineng.2019.04.009
Acknowledgements
We gratefully acknowledge the support received from the Yunnan Major Scientific and Technological Projects (No. 202202AG050011 and No. 202202AG050007), Yunnan industrial talent project (YNQR-CYRC-2018-013) and the Yunnan young and middle-aged academic and technical leaders reserve talent project (202305AC160064).
Funding
Yunnan Major Scientific and Technological Projects, 202202AG050011, Hengwei Yan,202202AG050007, Hengwei Yan,Yunnan industrial talent project, YNQR-CYRC-2018-003, Hengwei Yan,Yunnan young and middle-aged academic and technical leaders reserve talent project, 202305AC160064, Hengwei Yan
Author information
Authors and Affiliations
Contributions
JW: Conceptualization, Methodology, Writing-review& editing. ZL: Methodology, Visualization, Supervision. WY: Methodology, Resources. HY: Methodology, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, J., Liu, Z., Yan, H. et al. Study on separation of N, F and Al from hazardous aluminum dross by alkaline roasting. J Mater Cycles Waste Manag 25, 2485–2497 (2023). https://doi.org/10.1007/s10163-023-01711-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01711-x