Abstract
Background
Long-term dialysis vintage is a predictor of persistent hyperparathyroidism (HPT) after kidney transplantation (KTx). Recently, preemptive kidney transplantation (PKT) has increased. However, the incidence, predictors, and clinical implications of HPT after PKT are unclear. Here, we aimed to elucidate these considerations.
Methods
In this retrospective cohort study, we enrolled patients who underwent PKT between 2000 and 2016. Those who lost their graft within 1 year posttransplant were excluded. HPT was defined as an intact parathyroid hormone (PTH) level exceeding 80 pg/mL or hypercalcemia unexplained by causes other than HPT. Patients were divided into two groups based on the presence of HPT 1 year after PKT. The primary outcome was the predictors of HPT after PKT, and the secondary outcome was graft survival.
Results
Among the 340 consecutive patients who underwent PKT, 188 did not have HPT (HPT-free group) and 152 had HPT (HPT group). Multivariate logistic regression analysis revealed that pretransplant PTH level (P < 0.001; odds ratio [OR], 5.480; 95% confidence interval [CI], 2.070–14.50) and preoperative donor-estimated glomerular filtration rate (P = 0.033; OR, 0.978; 95% CI, 0.957–0.998) were independent predictors of HPT after PKT. Death-censored graft survival was significantly lower in the HPT group than that in the HPT-free group (90.4% vs. 96.4% at 10 years, P = 0.009).
Conclusions
Pretransplant PTH levels and donor kidney function were independent predictors of HPT after PKT. In addition, HPT was associated with worse graft outcomes even after PKT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperparathyroidism (HPT) is a typical complication of chronic kidney disease (CKD) often associated with mortality and various complications [1]. Although successful kidney transplantation (KTx) improves HPT to some extent [2], it often persists and adversely affects clinical outcomes even after KTx [3]. Long-term dialysis vintage is one of the main predictors of persistent HPT after KTx. Krinap et al. demonstrated that long-term dialysis vintage was significantly associated with persistent HPT in 640 patients with KTx [4]. Yamamoto et al. also reported dialysis vintage as a predictor of posttransplant HPT in a study of 520 patients [5]. Preemptive kidney transplantation (PKT), KTx performed before the initiation of chronic dialysis, has recently increased [6]. PKT is a superior treatment option in terms of both patient and graft outcomes [6]. However, the incidence, predictors, and clinical implications of HPT after PKT are unclear. Therefore, we conducted a retrospective cohort study to elucidate these considerations.
Materials and methods
Study design and subjects
Consecutive patients who underwent PKT between January 2000 and December 2016 at the Japanese Red Cross Aichi Medical Center, Nagoya Daini Hospital (Nagoya, Japan) were included. Data were collected on July 31, 2022. PKT was indicated for patients with Stage 5 CKD with an estimated glomerular filtration rate (eGFR) less than 15 mL/min/1.73 m2. The exclusion criteria were as follows: patients who underwent chronic dialysis before KTx, those who underwent parathyroidectomy before KTx, those for whom data were lacking, those who had lost their kidney graft within 1 year of KTx, and those who were under 16 years of age at the time of KTx. HPT was defined as intact parathyroid hormone (PTH) levels of > 80 pg/mL or hypercalcemia unexplained by causes other than HPT 1 year after KTx; intact PTH level has been reported to be ≤ 80 pg/mL in over 98% of healthy individuals irrespective of vitamin D status in both PTH assays used in this study [7]. Hypercalcemia was defined as total serum calcium (Ca) levels > 10.5 mg/dL.
Patients who met the inclusion criteria were divided into two groups based on the presence or absence of HPT: the HPT-free group, comprising patients without HPT, and the HPT group, comprising patients with HPT 1 year after PKT. Each patients’ sex, age, body mass index (BMI), diabetes mellitus status (%), hypertension status(%), original disease of CKD (%), parathyroid gland size (mm) (the parathyroid gland size of the recipients was routinely measured by ultrasound [US] before KTx), number of human leukocyte antigen (HLA) mismatches, positivity of donor-specific HLA antibody (DSA), treatment with vitamin D supplementation and calcimimetics before PKT, eGFR and laboratory data before and 1 year post-PKT, mean blood pressure (MBP) 1 year after PKT, and graft survival were documented. In addition, Ca deposition and interstitial fibrosis and tubular atrophy (IFTA) in the kidney graft were assessed by reviewing the reports of protocol biopsy performed 1 h and 1 year after KTx. IFTA was graded by an experienced transplant pathologist according to the Banff classification system. The primary outcome was the predictor of HPT after PKT, and the secondary outcome was death-censored graft survival. Pretransplant blood sample analyses were performed on all patients within 3 months before KTx, and posttransplant blood sample analyses were performed every month for 1 year after KTx and every second month thereafter. All samples were collected from fasting patients. The values of serum Ca and intact PTH obtained from blood samples 1 year after PKT were used for patient enrollment and classification. This study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Measurements
Serum Ca and phosphorus (P) levels were measured using standard methods. Intact PTH levels were measured using the following second-generation immunoassays: an electrochemical luminescence immunoassay (SRL, Tokyo, Japan, www.srl-group.co.jp, reference range [10–65 pg/mL]) or an enzyme immunoassay (TOSOH Company, Tokyo, Japan, www.tosoh.co.jp, reference range [9–80 pg/mL]). When serum albumin values were < 4.0 g/dL, all serum Ca values were corrected for serum albumin values as follows [8]:
The eGFR was evaluated using the creatinine equation provided by the Japanese Society of Nephrology [9].
Immunosuppression
Immunosuppressive regimens included calcineurin inhibitors (cyclosporine or tacrolimus), mycophenolic acid, mizoribine, everolimus, and glucocorticoids. Basiliximab was used as induction therapy. In addition, rituximab administration or splenectomy was used as induction therapy in anti-donor antibody-positive patients after KTx, except in those with low antibody titers.
Statistical analysis
Pearson’s Chi-square test was used to analyze nominal variables, and the Mann–Whitney U test was used for continuous variables. All results are presented as median (interquartile range [IQR]) because of their non-normal distribution, as confirmed by the Shapiro–Wilk normality test and histogram. The Spearman’s rank correlation coefficient was used to evaluate the correlations among the variables (Online Resource 1). Multivariate logistic regression analysis was performed to identify predictors of persistent HPT after PKT. Age [10], sex [11], diabetes mellitus [12], parathyroid gland size [13], serum Ca [14], P [15], and PTH levels [16] before PKT were included as covariates in the multivariate analysis; these factors have been associated with HPT in previous studies. In addition, preoperative donors’ eGFR, BMI, and hypertension were included in the multivariate analysis because of the heterogeneity between the HPT-free and HPT groups. Crude and multivariate-adjusted odds ratios (ORs) for HPT after PKT with intact PTH levels and preoperative donor eGFR categorized by tertiles were also examined. Kaplan–Meier survival curves and log-rank tests were used to estimate graft survival. Cox proportional hazards regression analysis was performed to evaluate the risk of death-censored graft loss. To assess the impact of HPT after PKT on graft survival, propensity score (PS) matching in a 1:1 ratio between the HPT-free and HPT groups was performed (Online Resources 2 and 3). A logistic regression model involving ten covariates was used to derive the PSs. These covariates included six continuous variables (recipient age, BMI, serum P, serum Ca, recipient eGFR, and MBP 1 year after KTx) and four nominal variables (recipient sex, ABO blood type incompatibility, diabetes mellitus, and preformed DSA). For sensitivity analysis, stratified hazard ratios (HRs) of HPT for graft loss were also analyzed using recipient age, BMI, serum Ca level, serum P level, MBP, ABO blood type, and sex (Online Resource 4). SPSS version 23.0 (IBM Corp., Armonk, NY, USA) and EZR version 1.40 [17] were used for the statistical analyses. Statistical significance was set at P < 0.05.
Results
Patient characteristics
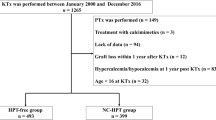
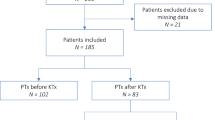
A total of 340 patients met the inclusion criteria (median observation period, 113 months [IQR, 86–146 months]). Of 340 patients, 188 and 152 were assigned to the HPT-free and HPT groups, respectively (Fig. 1). Patient characteristics are presented in Table 1. Significant differences were observed between both groups in donor age, donor hypertension, intact PTH level before PKT, and preoperative donor eGFR. In addition, serum Ca, intact PTH, and recipient eGFR 1 year after PKT were significantly different between the two groups. Other characteristics did not differ between the two groups (Table 1). Among the HPT group, two received parathyroidectomy and four received bisphosphonate treatment after PKT. None of the patients were administered calcimimetics during the follow-up period.
Predictors of HPT after PKT
The univariate logistic regression analysis demonstrated that log-intact PTH levels before PKT (P < 0.001; OR, 5.450; 95% confidence interval [CI] 2.380–12.50), donor age (P = 0.006; OR, 1.030; 95% CI 1.010–1.060), donor hypertension (P = 0.013; OR, 1.910; 95% CI 1.140–3.200), and preoperative donor eGFR (P = 0.002, OR, 0.971; 95% CI 0.953–0.989) were significantly associated with HPT after PKT (Table 2). Multivariate logistic regression analysis revealed that log-intact PTH before PKT (P < 0.001; OR, 5.500; 95% CI 2.090–14.50) and preoperative donor eGFR (P = 0.034; OR, 0.977; 95% CI 0.957–0.998) were significantly associated with HPT after PKT (Table 2). Figure 2 shows the crude and multivariate-adjusted ORs for HPT after PKT associated with the categories of intact PTH before PKT and the preoperative donor eGFR values. The multivariate-adjusted OR for HPT after PKT significantly increased when intact PTH levels exceeded 404.0 pg/mL (OR, 2.360; 95% CI 1.280–4.330) (Fig. 2a). Conversely, a significant increase in the multivariate adjusted OR of HPT after PKT was observed when preoperative donor eGFR was lower than 68.1 mL/min/1.73 m2 (OR, 1.830; 95% CI 1.010–3.300) (Fig. 2b).
ORs and 95% CIs for HPT after PKT according to the categories of pretransplant intact PTH levels and preoperative donor eGFR using crude and multivariable-adjusted logistic models. a ORs according to the categories of pretransplant intact PTH levels. The multivariable-adjusted analysis included recipient age, recipient sex, diabetes mellitus, pretransplant serum Ca and P levels, parathyroid gland size, donor age, donor sex, and preoperative donor eGFR. b ORs according to categories of preoperative donor eGFR. The multivariable-adjusted analysis included recipient age, recipient sex, diabetes mellitus, pretransplant serum intact PTH, Ca and P levels, parathyroid gland size, donor age, and donor sex. *P < 0.05. Ca calcium, 95% CI 95% confidence interval, eGFR estimated glomerular filtration rate, HPT hyperparathyroidism, OR odds ratio, PKT preemptive kidney transplantation, PTH parathyroid hormone
Graft survival
Graft loss was observed in 28 patients (6.3% vs. 10.7% in the HPT-free and HPT groups, respectively) (Table 1). Death-censored graft survival in the HPT group was significantly lower than that in the HPT-free group (90.4% vs. 96.4% at 10 years, P = 0.006) (Fig. 3a). Even after PS matching of the 114 recipients from each group, the death-censored graft survival of HPT recipients was inferior to that of HPT-free recipients (91.7% vs. 96.5% at 10 years, P = 0.031) (Fig. 3b). Univariate Cox proportional hazards analysis revealed that HPT after PKT was significantly associated with death-censored graft loss (P = 0.009; HR, 2.840; 95% CI 1.304–6.182) (Table 3). In addition, the Cox proportional hazards model adjusted by PS matching revealed significantly higher risk of death-censored graft loss in the HPT group than that in the HPT-free group (P = 0.039; HR, 3.047; 95% CI 1.057–8.787) (Table 3). Even in the stratified analysis, although it was not necessarily statistically significant in all strata due to a decrease in the number of events in each group, HPT tended to increase the risk of graft loss overall (Online Resource 4).
Graft biopsy findings
Protocol graft biopsies were performed in 335 and 325 patients 1 h, and 1 year after PKT, respectively. There was no significant difference in Ca deposition or IFTA between the two groups at 1 h post-PKT. However, both the incidence of Ca deposition and the severity of IFTA were significantly higher in the HPT group than those in the HPT-free group in kidney grafts 1 year post-PKT (Table 4).
Discussion
We hypothesized that the incidence of persistent HPT after PKT would be considerably lower because there is no dialysis vintage. However, high PTH levels were observed in over 40% of the patients even 1 year after PKT in the current study. Multivariate logistic regression analysis demonstrated that pretransplant PTH level and preoperative donor eGFR were predictors of HPT after PKT. In contrast, parathyroid gland size was not a predictor of HPT after PKT in the present study, which was inconsistent with previous reports [13]. This discrepancy is likely due to the limited accuracy of US imaging of the parathyroid glands [18].
The association between high pretransplant PTH levels and HPT after PKT demonstrated in the current study is consistent with previous reports in patients who underwent KTx, not limited to PKT. Kirnap et al. reported that intact PTH levels are a risk factor for persistent HPT after KTx [4]. Another study by Yamamoto et al. showed that intact pretransplant PTH levels correlated with HPT after KTx [5]. In addition, Sutton et al. demonstrated that intact PTH levels of > 300 pg/mL before KTx were associated with the development of HPT after KTx [16]. These findings highlight the importance of managing CKD mineral and bone disorders before KTx to prevent persistent HPT.
To the best of our knowledge, the significant association observed between a lower preoperative donor eGFR and persistent HPT after KTx is a novel finding. Kidney graft function depends, to some extent, on donor GFR [19, 20]. In addition, vitamin D status is associated with GFR [21] and plays a central role in HPT [22]. Taken together, it is reasonable to conclude that a low donor GFR contributes to the progression of posttransplant HPT through low kidney graft function and vitamin D deficiency. In other words, sufficient donor kidney function is important to prevent HPT after KTx.
In previous reports, posttransplant HPT was associated with poor kidney graft outcomes [3, 23, 24]. In this study, which focused only on patients who underwent PKT, graft survival was lower in patients with HPT than that in patients who were HPT-free. Furthermore, the inferiority in graft survival of the HPT group did not change even after PS matching for several known risk factors. This result indicates the importance of posttransplant monitoring of PTH and the recognition of persistent HPT as a risk factor for graft loss, regardless of dialysis vintage. Therefore, the necessity of posttransplant HPT treatment should be discussed even after PKT. Although the mechanisms by which HPT worsens graft survival are unclear, excess PTH has been associated with pathological fibrosis [25] and renal/vascular calcification [26,27,28]. In the current study, both the incidence of Ca deposition and the severity of IFTA on kidney grafts were higher in the HPT group than those in the HPT-free group, which may have affected long-term graft survival. Compared to the HPT-free group, the serum Ca levels were lower and the urine Ca levels were similar in the HPT group. In addition, most of the calcifications in the kidney grafts occurred in the epithelial cells and interstitium and not in the tubular lumen. Therefore, those lesions may be due to factors other than Ca concentration. Recently, various factors such as inflammatory cytokines, endoplasmic reticulum stress, autophagy dysfunction, mitochondrial dysfunction, and abnormal molecular signaling pathways have been reported to be involved in soft tissue calcification in addition to calcium and phosphorus concentrations. The mechanism of calcification of kidney grafts is unclear; however, it may be the result of the complex interaction of these multifactorial variables [29, 30].
This study has several limitations. First is its retrospective design. Second, it is a single-center study. Third is the inherent possibility of unmeasured confounders. Fourth, there may be selection bias. Fifth is the absence of data regarding endogenic vitamin D status, fibroblast growth factor 23, and other bone biomarkers. Further studies with larger sample sizes are required to validate our findings.
Conclusions
The incidence of persistent HPT 1 year after PKT is 40%, and pretransplant PTH levels and donor eGFR are the predictors. Monitoring and management of PTH levels even after PKT are equally important because HPT is associated with poor graft survival.
Data availability
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–18.
Reinhardt W, Bartelworth H, Jockenhövel F, Schmidt-Gayk H, Witzke O, Wagner K, et al. Sequential changes of biochemical bone parameters after kidney transplantation. Nephrol Dial Transplant. 1998;13(2):436–42.
Pihlstrøm H, Dahle DO, Mjøen G, Pilz S, März W, Abedini S, et al. Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation. 2015;99(2):351–9.
Kirnap NG, Kirnap M, Sayin B, Akdur A, Bascil Tutuncu N, Haberal M. Risk factors and treatment options for persistent hyperparathyroidism after kidney transplantation. Transplant Proc. 2020;52(1):157–61.
Yamamoto T, Tominaga Y, Okada M, Hiramitsu T, Tsujita M, Goto N, et al. Characteristics of persistent hyperparathyroidism after renal transplantation. World J Surg. 2016;40(3):600–6.
Goto N, Okada M, Yamamoto T, Tsujita M, Hiramitsu T, Narumi S, et al. Association of dialysis duration with outcomes after transplantation in a Japanese cohort. Clin J Am Soc Nephrol. 2016;11(3):497–504.
Yalla N, Bobba G, Guo G, Stankiewicz A, Ostlund R. Parathyroid hormone reference ranges in healthy individuals classified by vitamin D status. J Endocrinol Invest. 2019;42(11):1353–60.
Anonymous. Correcting the calcium. Br Med J. 1977,1 (6061): 598.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92.
Giusti A, Barone A, Razzano M, Pizzonia M, Oliveri M, Palummeri E, et al. High prevalence of secondary hyperparathyroidism due to hypovitaminosis D in hospitalized elderly with and without hip fracture. J Endocrinol Invest. 2006;29(9):809–13.
Sayed-Hassan R, Abazid N, Alourfi Z. Relationship between 25-hydroxyvitamin D concentrations, serum calcium, and parathyroid hormone in apparently healthy Syrian people. Arch Osteoporos. 2014;9:176.
Vincenti F, Hattner R, Amend WJ Jr, Feduska NJ, Duca RM, Salvatierra O Jr. Decreased secondary hyperparathyroidism in diabetic patients receiving hemodialysis. JAMA. 1981;245(9):930–3.
Jäger MD, Serttas M, Beneke J, Müller JA, Schrem H, Kaltenborn A, et al. Risk-factors for nodular hyperplasia of parathyroid glands in sHPT patients. PLoS ONE. 2017;12(10):e0186093.
Dewberry LC, Tata S, Graves S, Weber CJ, Sharma J. Predictors of tertiary hyperparathyroidism: Who will benefit from parathyroidectomy? Surgery. 2014;156(6):1631–6.
Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–8.
Sutton W, Chen X, Patel P, Karzai S, Prescott JD, Segev DL, et al. Prevalence and risk factors for tertiary hyperparathyroidism in kidney transplant recipients. Surgery. 2022;171(1):69–76.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Mihai R, Simon D, Hellman P. Imaging for primary hyperparathyroidism–an evidence-based analysis. Langenbecks Arch Surg. 2009;394(5):765–84.
Jinfeng L, Jia L, Tao G, Wenjun S, Xinlu P, Yonghua F, et al. Donor kidney glomerular filtration rate and donor/recipient body surface area ratio influence graft function in living related kidney transplantation. Ren Fail. 2015;37(4):576–81.
Zheng Y, Guan Y, Li Z, Zhang G, Guo Y, Ai X. Roles of donor/recipient body surface area ratio and donor kidney glomerular filtration rate in kidney selection for living transplantation from family members. Rev Invest Clin. 2018;70(4):169–76.
Ureña-Torres P, Metzger M, Haymann JP, Karras A, Boffa JJ, Flamant M, et al. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis. 2011;58(4):544–53.
Nakano C, Hamano T, Fujii N, Matsui I, Tomida K, Mikami S, et al. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol. 2012;7(5):810–9.
Araujo M, Ramalho JAM, Elias RM, Jorgetti V, Nahas W, Custodio M, et al. Persistent hyperparathyroidism as a risk factor for long-term graft failure: the need to discuss indication for parathyroidectomy. Surgery. 2018;163(5):1144–50.
Okada M, Tominaga Y, Sato T, Tomosugi T, Futamura K, Hiramitsu T, et al. Elevated parathyroid hormone one year after kidney transplantation is an independent risk factor for graft loss even without hypercalcemia. BMC Nephrol. 2022;23:212.
Panizo S, Martínez-Arias L, Alonso-Montes C, Cannata P, Martín-Carro B, Fernández-Martín JL, et al. Fibrosis in chronic kidney disease: pathogenesis and consequences. Int J Mol Sci. 2021;22(1):408.
Amaral LM, Queiroz DC, Marques TF, Mendes M, Bandeira F. Normocalcemic versus Hypercalcemic Primary Hyperparathyroidism: More Stone than Bone? J Osteoporos. 2012;2012:128352.
Okada M, Hiramitsu T, Ichimori T, Goto N, Narumi S, Watarai Y, et al. Comparison of pre- and post-transplant parathyroidectomy in renal transplant recipients and the impact of parathyroidectomy timing on calcium metabolism and renal allograft function: a retrospective single-center analysis. World J Surg. 2020;44(2):498–507.
Salam S, Gallagher O, Gossiel F, Paggiosi M, Eastell R, Khwaja A. Vascular calcification relationship to vascular biomarkers and bone metabolism in advanced chronic kidney disease. Bone. 2021;143:115699.
Lee SJ, Lee IK, Jeon JH. Vascular calcification—new insights into its mechanism. Int J Mol Sci. 2020;21:2685.
Pan W, Jie W, Huang H. Vascular calcification: Molecular mechanisms and therapeutic interventions. MedComm. 2020;2023(4):e200.
Acknowledgements
The authors thank Mr. Taiki Yamanaka of the Division of Medical Statistics at the Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital for valuable data collection and Editage (www.editage.com) for English language editing.
Funding
This study received no funding or financial support from any other industry.
Author information
Authors and Affiliations
Contributions
MO: conception, design, and drafting of the article; TS: conception and drafting of the article; YH, KF, TH, TI, and NG: acquisition and interpretation of data; SN, and AT: critical revision of the article for important intellectual content; YW: final approval of the manuscript for publication. All authors have consented to the publication of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board (IRB) of the Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital (IRB approval number: 1471) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
An informed consent waiver was obtained from the IRB of Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital. Details regarding the study and its opt-outs are provided in detail on our institutional website.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Okada, M., Sato, T., Hasegawa, Y. et al. Persistent hyperparathyroidism after preemptive kidney transplantation. Clin Exp Nephrol 27, 882–889 (2023). https://doi.org/10.1007/s10157-023-02371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-023-02371-9