Abstract
Background
In the era of combination therapy, there has been limited research on body composition. Specific body composition, such as sarcopenia, possesses the potential to serve as a predictive biomarker for toxic effects and clinical response in patients with urothelial carcinoma (UC) undergoing tislelizumab combined with gemcitabine and cisplatin (T + GC).
Materials and Methods
A total of 112 UC patients who received T + GC were selected at the Affiliated Hospital of Xuzhou Medical University from April 2020 to January 2023. Baseline patient characteristics and detailed hematological parameters were collected using the electronic medical system and laboratory examinations. The computed tomography images of patients were analyzed to calculate psoas muscle mass index (PMI). We evaluated the association between sarcopenia (PMI < 4.5 cm2/m2 in men; PMI < 3.3 cm2/m2 in women) and both hematological toxicity and tumor response.
Results
Overall, of the 112 patients (65.2% male, median age 56 years), 43 (38.4%) were defined as sarcopenia. Patients with sarcopenia were notably older (p = 0.037), more likely to have hypertension (p = 0.009), and had poorer ECOG-PS (p = 0.027). Patients with sarcopenia were more likely to develop leukopenia (OR 2.969, 95% CI 1.028–8.575, p = 0.044) after receiving at least two cycles of T + GC. However, these significant differences were not observed in thrombocytopenia and anemia. There were no significant differences in the tumor response and grade 3–4 hematological toxicity between patients with sarcopenia and those without sarcopenia.
Conclusions
Patients with sarcopenia were more likely to develop leukopenia after receiving T + GC. There were no notable alterations observed in relation to anemia or thrombocytopenia. No significant difference was found between the sarcopenia group and non-sarcopenia group in terms of tumor response and grade 3–4 hematological toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the advent of immune checkpoint inhibitors (ICIs), patients with locally advanced and metastatic urothelial carcinoma (UC) have a broader range of treatment options. Therefore, an increasing number of studies focus on the therapeutic effects of immunotherapy combined with chemotherapy. It is worth mentioning that the adverse effects (AEs) of combination therapy cannot be ignored. This underscores the urgent need for research into predictive and easily measurable biomarkers to identify suitable patients with UC for this treatment approach.
Sarcopenia is the most frequently assessed parameter for evaluating complex body composition and has significant clinical value. Sarcopenia refers to the loss of skeletal muscle mass and function, which is believed to be linked to perioperative complications [1, 2] and a negative prognosis in UC [3,4,5,6,7]. However, whether sarcopenia has an effect on treatment-related adverse events (TRAEs), particularly hematological toxicity, in UC patients receiving immunochemotherapy remains a subject of debate. Hence, we conducted a retrospective study to determine whether there is an association between pre-existing sarcopenia in UC patients before receiving immunochemotherapy and the incidence of treatment-related hematological toxicity as well as tumor response rates.
Materials and methods
Patients selection
From April 2020 to January 2023, 112 patients with UC who received first-line tislelizumab plus gemcitabine and cisplatin (T + GC) therapy at our institution were retrospectively evaluated. Eligible patients had histologically confirmed UC of the renal pelvis, ureter, or bladder and received at least two cycles of T + GC. Adequate laboratory examinations, including baseline hematological parameters and post-treatment complete blood count (CBC), were required.
Exclusion criteria included the inability to obtain computed tomography (CT) imaging. Patients who had previously received treatment with ICIs or cytotoxic chemotherapy should be excluded. Patients who had received prophylactic corticosteroids or other medications to promote hematopoiesis within 1 month prior to T + GC administration should also be excluded. A total of 112 patients met criteria (Fig. 1).
Ethical approval was granted by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (approval number: XYFY2022-KL340). All patients provided written informed consent.
Definition of sarcopenia
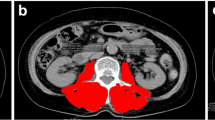
CT has been established as a reliable method for accurately measuring of body composition parameters [8], such as skeletal muscle and subcutaneous adipose tissue. We utilized the psoas muscle mass index (PMI) at the level of the third lumbar vertebra (L3) in CT images as an alternative indicator for evaluating skeletal muscle mass. The cross-sectional area (CSA) of the bilateral psoas muscles was calculated, and the mean value for two consecutive images was computed for each patient. All images were analyzed by two independent researchers using ImageJ software (Version 1.49, National Institutes of Health, USA) based on the aforementioned criteria. PMI (cm2/m2) was calculated using this formula: the mean CSA of psoas muscle (cm2) from two consecutive CT images / the square of the body height (m2).
A study based on a large sample of the Asian population demonstrated that the PMI cut-off value for sarcopenia in male patients was 6.36 cm2/m2, while in female patients it was 3.92 cm2/m2 [9]. In this study, sarcopenia was defined as less than 2 standard deviations (SDs) below the mean PMI of healthy Asian adults. However, our research focused on patients diagnosed with UC who were predominantly of older age, and almost all of them were classified as sarcopenia according to the above definition. Therefore, we adopted the method of selecting cut-off values for sarcopenia from previous studies [10] and utilized a cut-off value of > 1 SD below our mean value (< 4.5 cm2/m2 for male patients; < 3.3 cm2/m2 for female patients) to identify individuals with sarcopenia.
Data collection and evaluations
The clinicopathological data were collected from the medical records, including age, sex, body mass index (BMI), smoking history, hypertension, diabetes, hydronephrosis, Eastern Cooperative Oncology Group performance score (ECOG PS), primary tumor site (renal pelvis/ureter/bladder) and renal function. The CT scan within three months before the start of T + GC and throughout treatment should be collected.
The laboratory parameters include white blood cell (WBC) count, hemoglobin level, and platelet count. Patients with renal insufficiency need to meet both criteria of blood creatinine > 133 μmol/l and eGFR > 60 ml/min. The incidence and severity of TRAEs were assessed by Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03 of the National Cancer Institute.
Drug administration and the evaluation of responses
The patients received a 21-day cycle of treatment. Patients were administered intravenously gemcitabine (1000 mg per square meter of body-surface area) on days 1 and 8, in combination with cisplatin (70 mg per square meter of body-surface area) and tislelizumab (200 mg) on day 1 as first-line therapy. According to the pathological stages, the treatment cycle of T + GC varied. For patients with locally advanced UC, four cycles of postoperative adjuvant therapy were administered. For patients with metastatic UC, six cycles of systemic therapy were recommended. The combination therapy was terminated permanently until the completion of treatment schedule, disease progression, intolerable toxicity, or withdrawal of consent.
Response evaluation of the target lesions was performed via CT scan using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [11]. The first response evaluation was performed after two cycles of T + GC, followed by a reassessment every two treatment cycles.
Statistical analyses
All the data were transformed into categorical variables, and descriptive statistics, such as percentages, means, and medians were used to report baseline characteristics of patients. After testing for normal distribution, the Chi-square test and Fisher's exact test were used to investigate the differences between the sarcopenia group and the non-sarcopenia group. For non-normally distributed variables, the Wilcoxon–Mann–Whitney U test was performed. Univariate and multivariate logistic regression were conducted to examine potential risk factors. The results were reported as odds ratios (OR) with 95% confidence intervals (CI). All analyses were performed using SPSS 26.0 (IBM Corp., NY, USA). All statistical analyses were two-sided, and statistical significance was set at p < 0.05.
Results
Descriptive data
From April 2020 to January 2023, a total of 112 patients with UC received at least two cycles of T + GC. The patient characteristics are shown in Table 1. The median age was 56 (range 34–82), with 39 (34.8%) females and 73 (65.2%) males. Among the study population, 38.4% of patients were classified as sarcopenia. The age of patients with sarcopenia exhibited a significant increase (p = 0.037), and the median age of patients in the sarcopenia group and non-sarcopenia group was 63 years and 54 years, respectively. Additionally, patients with sarcopenia were more likely to have hypertension (p = 0.009), and had poorer ECOG PS (p = 0.027). Patients with sarcopenia were not associated with a significant reduction in BMI.
Sarcopenia and hematological toxicity
The majority of patients (n = 79; 70.5%) developed anemia during treatment. Among them, 47 (59.5%) patients developed anemia immediately after the first cycle of T + GC. After receiving one cycle of T + GC, patients with sarcopenia (n = 24; 55.8%) were more likely to develop anemia than those without sarcopenia (n = 23; 33.3%). Among patients receiving adjuvant treatment after radical surgery, 30 (75.0%) patients in the sarcopenia group experienced postoperative anemia, whereas 44 (67.7%) patients in the non-sarcopenia group developed anemia. None of the patients underwent dose adjustment due to anemia. One patient permanently discontinued treatment due to grade 4 anemia and received blood transfusion therapy. Patients with sarcopenia were more likely to develop grade 3–4 anemia (25.6 vs. 11.6%, p = 0.055), but this did not reach statistical significance (Table 2). Multivariate logistic regression analysis showed that the occurrence of anemia was associated with the age (OR 7.921, 95% CI 1.078–58.185, p = 0.042) of UC patients (Table 3).
Of the 112 UC patients who received T + GC, 44 (39.3%) developed thrombocytopenia. Compared to patients who did not undergo surgical treatment (n = 3; 2.7%), patients who underwent radical surgery were more likely to develop thrombocytopenia (n = 21; 18.8%) after multiple cycles of T + GC. Compared with the non-sarcopenia group, patients with sarcopenia were more likely to develop grade 3–4 thrombocytopenia (18.7 vs. 10.1%, p = 0.201). However, this difference did not reach the threshold for statistical significance (Table 2). Two patients permanently discontinued T + GC therapy due to severe thrombocytopenia, and one patient received a platelet transfusion. Results from multivariate logistic regression analysis showed that smoking history (OR 3.294, 95% CI 1.022–10.615, p = 0.046) was associated with thrombocytopenia in patients (Table 4).
Of the 112 patients with UC, half of the patients (n = 56; 50%) experienced leukopenia during all cycles of T + GC. Among patients with leukopenia, there were 9 (16.1%) patients in the non-sarcopenia group and 13 (23.2%) patients in the sarcopenia group who had pre-existing renal insufficiency prior to the start of T + GC. 28 (25.0%) patients treated with short-acting granulocyte colony stimulating factor, the WBC count rebounded enough to tolerate the next cycle of T + GC, and three patients with sarcopenia permanently discontinued therapy due to severe leukopenia. A statistically insignificant higher incidence of grade 3 or 4 leukopenia occurred in the sarcopenia group (39.6 vs. 30.4%, p = 0.324) (Table 2). The results from multivariate logistic regression analysis showed that patients with sarcopenia were associated with leukopenia after receiving T + GC (OR 2.969, 95% CI 1.028–8.575, p = 0.044) (Table 5). For UC patients received T + GC, patients with sarcopenia were more likely to develop leukopenia.
Sarcopenia and clinical response
During the treatment period, a total of 45 patients with UC had at least one lesion that could be evaluated and measured (Table 6). Of the 45 patients treated with T + GC, 11(24.4%) exhibited a partial response, 19(42.2%) experienced stable disease, and only one patient (2.2%) achieved a complete response. The objective response rate (ORR) and disease control rate (DCR) in the entire cohort were 26.7% and 68.9%, respectively. Patients with sarcopenia had a higher ORR than those without sarcopenia, although the difference did not reach statistical significance (31.3 vs. 24.1%, p = 0.606). The DCR in the sarcopenia group and non-sarcopenia group was 68.8% and 68.9%, respectively.
Discussion
Since the 1980s, cisplatin-based chemotherapy has been the first-line standard regimen for metastatic UC [12]. The European Association of Urology (EAU) guidelines recommended neoadjuvant chemotherapy (NAC) for T2-T4aN0M0 bladder cancer. If NAC is not performed, adjuvant chemotherapy (AC) is required [13]. For patients with advanced upper tract urothelial carcinoma (UTUC), postoperative AC is recommended [14]. However, despite effective treatment, the prognosis of muscle-invasive UC remains poor with 5-year survival rates less than 60% [15, 16]. The standard first-line chemotherapy in the treatment of muscle-invasive UC provides only a modest survival benefit.
With the emergence of ICIs, patients with locally advanced and metastatic UC have more therapeutic options. The Phase III trial, IMvigor130 [17], demonstrated that immunotherapy combined with chemotherapy, compared to first-line platinum-based chemotherapy, significantly improved progression-free survival (PFS) for patients with UC (p = 0.007). The CheckMate-901 trial revealed that for patients with unresectable or metastatic UC, the combination of nivolumab with cisplatin-based chemotherapy followed by nivolumab monotherapy demonstrated statistically significant benefits in OS and PFS compared to first-line cisplatin-based chemotherapy. In April 2020, tislelizumab was approved for the treatment of advanced UC with high expression of programmed death-ligand 1 (PD-L1), marking the beginning of a new era in immunotherapy for UC in China. Current researches indicate that in the neoadjuvant [18] or adjuvant treatment [19] of locally advanced or metastatic bladder cancer, the combination of T + GC has yielded satisfactory clinical outcomes. Therefore, there is an urgent need to find easy-to-measure and predictive parameters that can predict the risk of toxic reactions and tumor response in advance.
Sarcopenia is a condition characterized by the systemic deterioration of muscle function and mass. It is observed in approximately 15–50% of patients aged 65 and older, and it is particularly prevalent in patients with advanced tumors [20]. Compared to the skeletal muscle index (SMI), the PMI is easier to obtain with sonography [21], making it commonly used as an alternative indicator for assessing sarcopenia [22]. Many studies have demonstrated that sarcopenia predominantly has a negative impact on the prognosis of UC patients who undergo radical resection or chemotherapy [23,24,25]. Furthermore, sarcopenia is thought to be associated with an increased incidence of postoperative complications [26, 27].
There is currently an ongoing debate regarding whether pre-existing sarcopenia prior to immunotherapy increases the risk of AEs. In patients with metastatic melanoma undergoing ICI therapy, those with sarcopenia experienced a higher incidence of grade ≥ 3 AEs compared to patients without sarcopenia, with anemia and fatigue being the most common AEs [28]. A meta-analysis revealed that there was no significant increase in the incidence of immune-related AEs (irAEs) in patients with sarcopenia after receiving immunotherapy [29]. In patients with unresectable metastatic UC undergoing ICI therapy, there was no significant correlation between sarcopenia status and the incidence of irAEs (univariate analysis, p = 0.1) [30]. The researchers postulated that patients without sarcopenia might exhibit higher immune activity, thereby rendering them more susceptible to the occurrence of irAEs. There is also no consensus on tumor response. Among advanced UC patients treated with ICI, the ORR was significantly higher in those without sarcopenia compared to those with sarcopenia (p = 0.019) [31]. However, according to Klatte et al., there was no association between pre-NAC sarcopenia and tumor response (p = 0.65) in muscle-invasive bladder cancer patients [32].
To our knowledge, this study is the first to focus on the relationship between pre-existing sarcopenia in UC patients receiving T + GC and hematological toxicity as well as tumor response. In this study, a total of 112 patients with UC underwent T + GC therapy for at least two cycles. Patients with sarcopenia were older, more likely to have hypertension, and had poorer ECOG-PS. In addition, we observed that patients with sarcopenia were more prone to experiencing leukopenia following T + GC therapy. At present, we have not been able to fully ascertain the potential reasons for this correlation and additional research is needed to explain this discrepancy. In terms of tumor response, we did not observe a statistically significant difference between the group of patients with sarcopenia and the group without sarcopenia. This may be due to the small sample size. Assessing patients' body composition is of significant relevance in guiding clinicians to select those who are most likely to achieve minimal hematological toxicity when considering T + GC therapy. For patients with sarcopenia, clinicians should monitor blood routine, particularly WBC count, more frequently to promptly adjust medication dosages and avoid treatment-related severe toxicities. Grade 3–4 hematological toxicity can be prevented by regular monitoring or therapeutic interventions. Whether patients with pre-existing sarcopenia can potentially prevent the occurrence of high-grade hematological toxicity through appropriate dietary interventions and exercise is a focus of our future research.
Our study has some limitations. Firstly, due to the small sample size of the retrospective study from a single institution, especially in the subgroup analysis, selection bias is inevitable. Secondly, due to the multitude of measurement methods and indices for assessing sarcopenia, making it challenging to select an appropriate cut-off value, prospective multicenter studies are required to establish clear standards and tools in this regard. Thirdly, no clear data are available on the optimal schedule for administering tislelizumab and GC chemotherapy. Finally, tumor response rate does not always indicate the potential survival benefits for patients. However, due to limitations in sample size and follow-up duration, we are currently unable to accurately assess the impact of sarcopenia on PFS and OS in patients with UC.
Conclusion
Patients with sarcopenia were more likely to develop leukopenia after receiving at least two cycles of T + GC. No significant difference was found between the sarcopenia group and non-sarcopenia group in terms of tumor response and grade 3–4 hematological toxicity. For patients with sarcopenia who are prone to myelosuppression, we should fully inform them of the risks before the start of T + GC and be vigilant in monitoring blood tests closely.
Data availability
The dataset analyzed in this study is available from the corresponding author upon reasonable request.
References
Wan F, Zhu Y, Gu C et al (2014) Lower skeletal muscle index and early complications in patients undergoing radical cystectomy for bladder cancer. World J Surg Oncol 12:14
Smith A, Deal A, Yu H et al (2014) Sarcopenia as a predictor of complications and survival following radical cystectomy. J Urol 191(6):1714–1720
Psutka S, Carrasco A, Schmit G et al (2014) Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer 120(18):2910–2918
Taguchi S, Akamatsu N, Nakagawa T et al (2016) Sarcopenia evaluated using the skeletal muscle index is a significant prognostic factor for metastatic urothelial carcinoma. Clin Genitourin Cancer 14(3):237–243
Kasahara R, Kawahara T, Ohtake S et al (2017) A low psoas muscle index before treatment can predict a poorer prognosis in advanced bladder cancer patients who receive gemcitabine and nedaplatin therapy. Biomed Res Int 2017:7981549
Engelmann S, Pickl C, Haas M et al (2023) Body composition of patients undergoing radical cystectomy for bladder cancer: sarcopenia, low psoas muscle index, and myosteatosis are independent risk factors for mortality. Cancers 15(6)
Pickl C, Engelmann S, Girtner F et al (2023) Body composition as a comorbidity-independent predictor of survival following nephroureterectomy for urothelial cancer of the upper urinary tract. Cancers 15(2)
Mitsiopoulos N, Baumgartner R, Heymsfield S et al (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (Bethesda, MD: 1985) 85(1):115–122
Hamaguchi Y, Kaido T, Okumura S et al (2016) Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition (Burbank, Los Angeles County, Calif) 32:1200–1205
Kano M, Hihara J, Tokumoto N et al (2021) Association between skeletal muscle loss and the response to nivolumab immunotherapy in advanced gastric cancer patients. Int J Clin Oncol 26(3):523–531
Nishino M, Jackman D, Hatabu H et al (2010) New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. Am J Roentgenol 195(3):W221–W228
Bellmunt J, Petrylak DP (2012) New therapeutic challenges in advanced bladder cancer. Semin Oncol 39(5):598–607
Alfred Witjes J, Lebret T, Compérat E et al (2017) Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 71(3):462–475
Birtle A, Johnson M, Chester J et al (2020) Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet (London, England) 395(10232):1268–1277
Galsky M, Stensland K, Moshier E et al (2016) Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol 34(8):825–832
Grossman H, Natale R, Tangen C et al (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349(9):859–866
Galsky MD, Arija JÁA, Bamias A et al (2020) Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 395(10236):1547–1557
Hu J, Chen J, Ou Z et al (2022) Neoadjuvant immunotherapy, chemotherapy, and combination therapy in muscle-invasive bladder cancer: a multi-center real-world retrospective study. Cell Rep Med 3(11):100785
Ren X, Tian Y, Wang Z et al (2022) Tislelizumab in combination with gemcitabine plus cisplatin chemotherapy as first-line adjuvant treatment for locally advanced or metastatic bladder cancer: a retrospective study. BMC Urol 22(1):128
Fukushima H, Takemura K, Suzuki H et al (2018) Impact of sarcopenia as a prognostic biomarker of bladder cancer. Int J Mol Sci 19(10)
Hari A, Berzigotti A, Štabuc B et al (2019) Muscle psoas indices measured by ultrasound in cirrhosis—preliminary evaluation of sarcopenia assessment and prediction of liver decompensation and mortality. Dig Liver Dis 51(11):1502–1507
Stangl-Kremser J, Ahmadi H, Derstine B et al (2021) Psoas muscle mass can predict postsurgical outcomes in patients who undergo radical cystectomy and urinary diversion reconstruction. Urology 158:142–149
Mayr R, Gierth M, Zeman F et al (2018) Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle 9(3):505–513
Shimizu R, Honda M, Teraoka S et al (2022) Sarcopenia is associated with survival in patients with urothelial carcinoma treated with systemic chemotherapy. Int J Clin Oncol 27(1):175–183
Hirasawa Y, Nakashima J, Yunaiyama D et al (2016) Sarcopenia as a novel preoperative prognostic predictor for survival in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol 23:1048–1054
Mayr R, Fritsche H, Zeman F et al (2018) Sarcopenia predicts 90-day mortality and postoperative complications after radical cystectomy for bladder cancer. World J Urol 36(8):1201–1207
Cohen S, Gal J, Freifeld Y et al (2021) Nutritional status impairment due to neoadjuvant chemotherapy predicts post-radical cystectomy complications. Nutrients 13(12)
Daly L, Power D, O’Reilly Á et al (2017) The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer 116(3):310–317
Li S, Wang T, Lai W et al (2021) Prognostic impact of sarcopenia on immune-related adverse events in malignancies received immune checkpoint inhibitors: a systematic review and meta-analysis. Transl Cancer Res 10(12):5150–5158
Shimizu T, Miyake M, Hori S et al (2020) Clinical impact of sarcopenia and inflammatory/nutritional markers in patients with unresectable metastatic urothelial carcinoma treated with pembrolizumab. Diagnostics (Basel, Switzerland) 10(5)
Fukushima H, Fukuda S, Moriyama S et al (2020) Impact of sarcopenia on the efficacy of pembrolizumab in patients with advanced urothelial carcinoma: a preliminary report. Anticancer Drugs 31(8):866–871
Mari A, D’Andrea D, Kimura S et al (2018) Sarcopenia as a predictive factor for response to upfront cisplatin-based chemotherapy in patients with muscle-invasive urothelial bladder cancer. Urol Int 101(2):197–200
Acknowledgements
This work was supported by grants from the Six Talent Peaks Project in Jiangsu Province (Grant number WSW-064), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (Grant number 19KJB310001), the Post-doctoral Research Funding Project in Jiangsu Province (Grant number 2021K447C), the Second Round of Xuzhou Medical Leading Talents Training Project (Grant number XWRCHT20210027), the Science and Technology Planning Project of Traditional Chinese Medicine in Jiangsu (Grant number YB2020050), the Practice Innovation Program of Jiangsu Province (Grant number KYCX21_2641), and the Postgraduate Research and Practice Innovation Project of Jiangsu Province (Grant number KYCX23-2926).
Author information
Authors and Affiliations
Contributions
ZG and YP are the primary authors of this paper. ZG was primarily responsible for formulating the overall research objectives and drafting the initial manuscript, while YP was responsible for critically reviewing it. XQ and GL were primarily responsible for collecting experimental data. ZW and LZ were primarily responsible for using statistical software to visualize the data. JW and NQ were primarily responsible for supervising and leading the planning and implementation of the research activities. HL and NQ are the corresponding authors of this article. Li was responsible for securing financial support for this publication project. All the aforementioned authors have reviewed the manuscript and have consented to its publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
This retrospective single-institutional study was approved by the ethics committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2022-KL340). All patients provided written informed consent.
Consent for publication
Informed consent to publish was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Gao, Z., Pang, Y., Qin, X. et al. Sarcopenia is associated with leukopenia in urothelial carcinoma patients who receive tislelizumab combined with gemcitabine and cisplatin therapy. Int J Clin Oncol 29, 592–601 (2024). https://doi.org/10.1007/s10147-023-02448-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02448-1