Abstract
Background/Aim
Osteopontin (OPN) is a secretory glycoprotein, which is expressed not only in osteoblasts, but immune cells including macrophages and activated T cells. Its pleiotropic immune functions, such as bone remodeling, cancer progression, immune response, and inflammation have been reported previously. However, the association between OPN and postoperative complications (POC) after colorectal cancer (CRC) surgery has not been studied, so far.
Methods
Peripheral blood samples were collected before (pre) and immediately after surgery (post), and on postoperative days (POD) 1, 3, 5, and 7. Serum OPN levels were measured by ELISA. In total, 78 patients who underwent elective CRC surgery were divided into the No-POC (n = 54) and POC (n = 24) groups.
Results
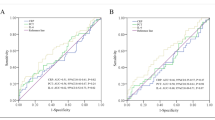
The POC group had significantly higher OPN levels than the No-POC group throughout the postoperative observation period. The maximum OPN levels from pre- to postsurgical samples showed the best predictive potential for POCs (cut off: 20.75 ng/mL, area under the curve: 0.724) and were correlated with length of postoperative stays. OPN values were significantly correlated with C-reactive protein on POD3 and were identified as an independent predictive marker for POCs (odds ratio: 3.88, 95% CI: 1.175–12.798, P = 0.026). The severity of POCs was reflected in increased OPN levels.
Conclusion
Increased postoperative OPN was associated with increased postoperative inflammatory host responses and POC after CRC surgery. Serum OPN level may be a useful biomarker for early prediction of POC and it may provide additional information for treatment decisions to prevent POC.






Similar content being viewed by others
References
Arnold M, Sierra MS, Laversanne M et al (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66:683–691
Torre LA, Siegel RL, Ward EM et al (2016) Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev 25:16–27
Oliphant R, Nicholson GA, Horgan PG et al (2014) The impact of surgical specialisation on survival following elective colon cancer surgery. Int J Colorectal Dis 29:1143–1150
Alves A, Panis Y, Mathieu P et al (2005) Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 140:278–283 (discussion 284)
Maruyama H, Kusachi S, Makino H et al (2020) Postoperative infection after colorectal surgery: subanalysis of data from the 2015 Japan postoperative infectious complications survey. J Nippon Med Sch 87:204–210
McSorley ST, Watt DG, Horgan PG et al (2016) Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol 23:2832–2840
Law WL, Choi HK, Lee YM et al (2007) The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol 14:2559–2566
Miyamoto Y, Hiyoshi Y, Tokunaga R et al (2020) Postoperative complications are associated with poor survival outcome after curative resection for colorectal cancer: a propensity-score analysis. J Surg Oncol 122:344–349
Wang KX, Denhardt DT (2008) Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev 19:333–345
Cantor H, Shinohara ML (2009) Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev Immunol 9:137–141
Lund SA, Giachelli CM, Scatena M (2009) The role of osteopontin in inflammatory processes. J Cell Commun Signal 3:311–322
Santamaria MH, Corral RS (2013) Osteopontin-dependent regulation of Th1 and Th17 cytokine responses in Trypanosoma cruzi-infected C57BL/6 mice. Cytokine 61:491–498
Hirano Y, Aziz M, Yang WL et al (2015) Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit Care 19:53
Aziz MM, Ishihara S, Mishima Y et al (2009) MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J Immunol 182:7222–7232
Cen C, Aziz M, Yang WL et al (2017) Osteopontin blockade attenuates renal injury after ischemia reperfusion by inhibiting NK cell infiltration. Shock 47:52–60
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240:205–213
Jain S, Chakraborty G, Bulbule A et al (2007) Osteopontin: an emerging therapeutic target for anticancer therapy. Expert Opin Ther Targets 11:81–90
Zhao H, Chen Q, Alam A et al (2018) The role of osteopontin in the progression of solid organ tumour. Cell Death Dis 9:356
Hattori T, Iwasaki-Hozumi H, Bai G et al (2021) Both full-length and protease-cleaved products of osteopontin are elevated in infectious diseases. Biomedicines 9(8):1006
Mishima R, Takeshima F, Sawai T et al (2007) High plasma osteopontin levels in patients with inflammatory bowel disease. J Clin Gastroenterol 41:167–172
Scatena M, Liaw L, Giachelli CM (2007) Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 27:2302–2309
Menger MD, Vollmar B (2004) Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg 389:475–484
Roumen RM, Hendriks T, van der Ven-Jongekrijg J et al (1993) Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg 218:769–776
Korner H, Nielsen HJ, Soreide JA et al (2009) Diagnostic accuracy of C-reactive protein for intraabdominal infections after colorectal resections. J Gastrointest Surg 13:1599–1606
Platt JJ, Ramanathan ML, Crosbie RA et al (2012) C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol 19:4168–4177
Warschkow R, Tarantino I, Torzewski M et al (2011) Diagnostic accuracy of C-reactive protein and white blood cell counts in the early detection of inflammatory complications after open resection of colorectal cancer: a retrospective study of 1,187 patients. Int J Colorectal Dis 26:1405–1413
Cardinale F, Chinellato I, Caimmi S et al (2011) Perioperative period: immunological modifications. Int J Immunopathol Pharmacol 24:S3-12
Lenz A, Franklin GA, Cheadle WG (2007) Systemic inflammation after trauma. Injury 38:1336–1345
Zhang X, Tang M, Zhang Q et al (2021) The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin Nutr 40:1224–1232
van Kooten RT, Bahadoer RR, Peeters K et al (2021) Preoperative risk factors for major postoperative complications after complex gastrointestinal cancer surgery: a systematic review. Eur J Surg Oncol 47:3049–3058
van Kooten RT, Voeten DM, Steyerberg EW et al (2022) Patient-related prognostic factors for anastomotic leakage, major complications, and short-term mortality following esophagectomy for cancer: a systematic review and meta-analyses. Ann Surg Oncol 29:1358–1373
Morimoto J, Kon S, Matsui Y et al (2010) Osteopontin; as a target molecule for the treatment of inflammatory diseases. Curr Drug Targets 11:494–505
Shao Z, Morser J, Leung LLK (2014) Thrombin cleavage of osteopontin disrupts a pro-chemotactic sequence for dendritic cells, which is compensated by the release of its pro-chemotactic C-terminal fragment. J Biol Chem 289:27146–27158
Agnihotri R, Crawford HC, Haro H et al (2001) Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 276:28261–28267
Ashkar S, Weber GF, Panoutsakopoulou V et al (2000) Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287:860–864
Chabas D, Baranzini SE, Mitchell D et al (2001) The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294:1731–1735
Da Silva AP, Pollett A, Rittling SR et al (2006) Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death. J Cell Physiol 208:629–639
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15K10037: Matsuda A). We thank James P. Mahaffey, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
AM: Study concept and design; KS, MY, NS: Acquisition of data; AM, SM, YK, TY: Analysis and interpretation of data; KS, AM: Drafting of the manuscript; MM, HY: Study supervision.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sekiguchi, K., Matsuda, A., Yamada, M. et al. The utility of serum osteopontin levels for predicting postoperative complications after colorectal cancer surgery. Int J Clin Oncol 27, 1706–1716 (2022). https://doi.org/10.1007/s10147-022-02225-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02225-6