Abstract
Background
In recent years, a better understanding of tumor biology and molecular features of gastric cancer has been reached. It may serve as a roadmap for patient stratification and trials of targeted therapies. The apparent efficacy of PD-1 blockade might be limited to a relatively small subset of advanced gastric cancer patients.
Materials and methods
In this study, preclinical and clinical studies, which investigated molecular features, promising treatment targets, and immune checkpoint inhibitor in gastric cancer, were reviewed via PubMed and the congress webpages of the American Society of Clinical Oncology and European Society of Medical Oncology.
Results
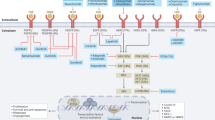
Next-generation sequencing technologies have defined the genomic landscape of gastric cancer. Indeed, several molecular classifications have been proposed, and distinct molecular subtypes have been identified. Based on these molecular profiles, clinical trials of new agents such as receptor tyrosine kinases inhibitors, antibody–drug conjugates, and IMAB362 (anti–Claudin 18.2) are ongoing. In addition, biomarkers to predict response during immune checkpoint inhibitors and combination therapy have been enthusiastically investigated.
Conclusion
Remarkable advances in an understanding of molecular profiles of gastric cancer enable the development of novel agents. The better treatment selection of immune checkpoint inhibitors or combination therapy should be established. These developments could facilitate precision medicine on gastric cancer in the near future.
Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. In J Cancer Journal international du cancer 136:e359–e386
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Wilke H, Muro K, Van Cutsem E et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235
Janowitz T, Thuss-Patience P, Marshall A et al (2016) Chemotherapy vs supportive care alone for relapsed gastric, gastroesophageal junction, and oesophageal adenocarcinoma: a meta-analysis of patient-level data. Br J Cancer 114:381–387
Fuchs CS, Tomasek J, Yong CJ et al (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31–39
Kang YK, Boku N, Satoh T et al (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390(10111):2461–2471
Shitara K, Doi T, Dvorkin M et al (2018) Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 19(11):1437–1448
The Cancer Genome Atlas Research Network (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202–209
Cristescu R, Lee J, Nebozhyn M et al (2015) Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 21:449–456
Li X, Wu WK, Xing R et al (2016) Distinct subtypes of gastric cancer defined by molecular characterization include novel mutational signatures with prognostic capability. Cancer Res 76:1724–1732
Kakiuchi M, Nishizawa T, Ueda H et al (2014) Recurrent gainof- function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet 46:583–587
Wong SS, Kim KM, Ting JC et al (2014) Genomic landscape and genetic heterogeneity in gastric adenocarcinoma revealed by whole-genome sequencing. Nat Commun 5:5477
Chen K, Yang D, Li X et al (2015) Mutational landscape of gastric adenocarcinoma in Chinese: implications for prognosis and therapy. Proc Natl Acad Sci USA 112:1107–1112
Deng N, Goh LK, Wang H et al (2012) A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61:673–684
Kwak EL, Ahronian LG, Siravegna G et al (2015) Molecular heterogeneity and receptor coamplification drive resistance to targeted therapy in METamplified esophagogastric cancer. Cancer Discov 5:1271–1281
Kuboki Y, Yamashita S, Niwa T et al (2016) Comprehensive analyses using next-generation sequencing and immunohistochemistry enable precise treatment in advanced gastric cancer. Ann Oncol 27:127–133
Nagatsuma AK, Aizawa M, Kuwata T et al (2015) Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer 18(2):227–238
Satoshi Y et al (2018) The nationwide cancer genome screening project in Japan SCRUM-Japan GI-SCREEN: Efficient identification of cancer genome alterations in advanced gastric cancer (GC). J Clin Oncol 36(15_suppl):4050
Kawazoe A, Shitara K, Kuboki Y et al (2019) Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein–Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer 22(1):69–76
Stahl P, Seeschaaf C, Lebok P et al (2015) Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol 4(15):7
Pectasides E, Stachler MD, Derks S et al (2018) Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov 8(1):37–48
Kim ST, Banks KC, Pectasides E et al (2018) Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol 29(4):1037–1048
Kato S, Okamura R, Baumqartner JM et al (2018) Analysis of circulating tumor dna and clinical correlates in patients with esophageal, gastroesophageal junction, and gastric adenocarcinoma. Clin Cancer Res 24(24):6248–6256
Riches JC, Schultz N, Ku GY et al (2015) Genomic profiling of esophagogastric (EG) tumors in clinical practice. J Clin Oncol 33(Suppl 3):57
Secrier M, Li X, de Silva N et al (2016) Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet 48:1131–1141
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Kim ST, Banks KC, Pectasides E et al (2018) Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol 29(4):1037–1048
Janjigian YY, Sanchez-Vega F, Jonsson P et al (2018) Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 8(1):49–58
Lee JY, Hong M, Kim ST et al (2018) The impact of concomitant genomic alterations on treatment outcome for trastuzumab therapy in HER2-positive. Gastric Cancer 29(4):1037–1048
Kang YK, Shah MA, Ohtsu A et al (2016) A randomized, open-label, multicenter, adaptive phase 2/3 study of trastuzumab emtansine (T-DM1) versus a taxane (TAX) in patients (pts) with previously treated HER2-positive locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma (LA/MGC/GEJC). J Clin Oncol 34(Suppl 4S):5
Ruschoff J, Hanna W, Bilous M et al (2012) HER2 testing in gastric cancer: a practical approach. Mod Pathol 25(5):637–650
Lee JY, Hong M, Kim ST et al (2015) The impact of concomitant genomic alterations on treatment outcome for trastuzumab therapy in HER2-positive gastric cancer. Sci Rep 5:9289
Press MF, Ellis CE, Gagnon RC et al (2017) HER2 status in advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma for entry to the TRIO-013/LOGiC trial of lapatinib. Mol Cancer Ther 16(1):228–238
Ogitani Y, Aida T, Hagihara K et al (2016) DS-8201a, a novel HER2-targeting ADC with a novel dna topoisomerase i inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 22(20):5097–5108
Doi T, Shitara K, Naito Y et al (2017) Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 18(11):1512–1522
Shitara K, Iwata H, Takahashi S et al (2017) Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol 18(11):1512–1522
Kwak EL, LoRusso P, Hamid O et al (2015) Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer [abstract]. J Clin Oncol 33(Suppl 3):1
Shitara K, Kim TM, Yokota T et al (2017) Phase I dose-escalation study of the c-Met tyrosine kinase inhibitor SAR125844 in Asian patients with advanced solid tumors, including patients with MET-amplified gastric cancer. Oncotarget 8(45):79546–79555
Smyth EC, Turner NC, Peckitt C et al (2015) Phase II multicenter proof of concept study of AZD4547 in FGFR amplified tumours [abstract]. J Clin Oncol 33(Suppl):2508
Pearson A, Smyth E, Babina IS et al (2016) High-level clonal FGFR amplification and response to FGFR inhibition in a translational clinical trial. Cancer Discov 6(8):838–851
Van Cutsem E, Bang YJ, Mansoor W et al (2017) A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann Oncol 28(6):1316–1324
Kuboki Y, Matsumura N, Bando H et al (2017) First-in-human (FIH) study of TAS-120, a highly selective covalent oral fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced solid tumors. Ann Oncol 28(suppl_5):v122–v141. https://doi.org/10.1093/annonc/mdx367
Sase H, Nakanishi Y, Aida S et al (2018) Acquired JHDM1D-BRAF fusion confers resistance to FGFR inhibition in FGFR2-amplified gastric cancer. Mol Cancer Ther 17(10):2217–2225
Kim SY, Ahn T, Bang H et al (2017) Acquired resistance to LY2874455 in FGFR2-amplified gastric cancer through an emergence of novel FGFR2-ACSL5 fusion. Oncotarget 8(9):15014–15022
Lordick F, Kang YK, Salman P et al (2013) Clinical outcome according to tumor HER2 status and EGFR expression in advanced gastric cancer patients from the EXPAND study. J Clin Oncol 31(15_suppl):4021
Maron SB, Alpert L, Kwak HA et al (2018) Targeted therapies for targeted populations: anti-EGFR treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov 8(6):696–713
Bang YJ, Im SA, Lee KW et al (2015) Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. J Clin Oncol 33:3858–3865
Bang YJ, Xu RH, Chin K et al (2017) Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 18(12):1637–1651
Liu Y, Hodgson D, Locker G et al (2018) Olaparib plus paclitaxel sensitivity in biomarker subgroups of gastric cancer. Ann Oncol 29(suppl_8):viii14–viii57
Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K et al (2015) Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci USA 112:1839–1844
Becerra C, Stephenson J, Jonker DJ et al (2015) Phase Ib/II study of cancer stem cell (CSC) inhibitor BBI608 combined with paclitaxel in advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol 33(15 Suppl):4069
Shah MA, Muro K, Shitara K et al. (2017) The BRIGHTER trial: a phase III randomized double-blind study of BBI608+ weekly paclitaxel versus placebo (PBO)+ weekly paclitaxel in patients (pts) with pretreated advanced gastric and gastro-esophageal junction (GEJ) adenocarcinoma. J Clin Oncol 36(15_suppl):4010
Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295:2387–2392
Shah MA, Starodub A, Sharma S et al (2018) Andecaliximab/GS-5745 alone and combined with mFOLFOX6 in advanced gastric and gastroesophageal junction adenocarcinoma: results from a Phase I study. Clin Cancer Res 24(16):3829–3837
Shah MA, Ruiz EY, Bodoky G et al (2019) A Phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of andecaliximab combined with mFOLFOX6 as first-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma (GAMMA-1). J Clin Oncol 37(4):4
Samuel JK, Johanna CB, Victoria MV, et al. safety and efficacy of a DKK1 inhibitor (DKN-01) in combination with pembrolizumab (P) in patients (Pts) with advanced gastroesophageal (GE) malignancies. Anna Oncol 29(8):viii205-viii270. https://doi.org/10.1093/annonc/mdy282
Niimi T, Nagashima K, Ward JM et al (2001) claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol 21(21):7380–7390
Sahin U, Koslowski M, Dhaene K et al (2008) Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 14(23):7624–7634
Tanaka A, Ishikawa A, Ushiku T et al (2018) Frequent CLDN18-ARHGAP fusion in highly metastatic diffuse-type gastric cancer with relatively early onset. Oncotarget 9(50):29336–29350
Singh P, Toom S, Huang Y (2017) Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol 10(1):105
Trarbach T, Schuler M, Zvirbule Z et al (2014) Efficacy and safety of multiple doses of IMAB362 in patients with advanced gastroesophageal cancer: results of a phase II study. Ann Oncol 25(4 Supplement):218
Al-Batran SE, Schuler MH, Zvirbule Z et al (2016) FAST: an international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol 34:4001
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454
Robert C, Long GV, Brady B et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Charles SF, Doi T, Raymond WJJ et al (2017) KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 35(15_suppl):4003
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Bang YJ, Ruiz EY, Van Cutsem E et al (2018) Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 29(10):2052–2060
Shitara K, Ozguroglu M, Bang YJ et al (2018) Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392:123–133
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520
Le DT, Durham JN, Smith KN et al (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409–413
Diaz LA Jr, Marabelle A, Delord JP et al (2017) Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. J Clin Oncol 35(15 suppl):3071
Llosa NJ, Cruise M, Tam A et al (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5(1):43–51
Schellens JHM, MaraBelle A, Zeigenfuss S et al (2017) Pembrolizumab for previously treated advanced cervical squamous cell cancer: Preliminary results from the phase 2 KEYNOTE-158 study. J Clin Oncol 35(15_suppl):5514
Muro K, Van Cutsem E, Narita Y et al (2019) Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer; a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 30(1):19–33
Kim ST, Cristescu R, Bass AJ et al (2018) Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 24(9):1449–1458
Panda A, Mehnert JM, Hirshfield KM et al (2017) Immune activation and benefit from avelumab in EBV-positive gastric cancer. J Natl Cancer Inst 110(3):316–320
Mishima S, Kawazoe A, Nakamura N et al. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immun Cancer 7(1):24
Fukuoka S, Motooka D, Togashi Y et al (2018) Association of gut microbiome with immune status and clinical response in solid tumor patients who received on anti-PD-1 therapies. J Clin Oncol 36(15_suppl):3011
Kang YK, Satoh T, Chao Y (2019) Evaluation of Efficacy of Nivolumab by Baseline Factors from ATTRACTION-2. J Clin Oncol 37(suppl 4):8
Champiat S, Dercle L, Ammari S et al (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23:1920–1928
Saada-Bouzid E, Defaucheux C, Karabajakian A et al (2017) Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 28:1605–1611
Kato S, Goodman A, Walavalkar V et al (2017) Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 23:4242–4250
Kurman JS, Murgu SD (2018) Hyperprogressive disease in patients with non-small cell lung cancer on immunotherapy. J Thoracic Dis 10:1124–1128
Sasaki A, Nakamura Y, Mishima S et al (2018) Predictive factors for hyperprogression during nivolumab treatment in patients with advanced gastric cancer. Gastric Cancer. https://doi.org/10.1007/s10120-018-00922-8
Togashi Y KT, Sasaki A et al (2018) Clinicopathological, genomic and immunological features of hyperprogressive disease during PD-1 blockade in gastric cancer patients. J Clin Oncol 36(15 suppl):4106
Lo Russo G, Moro M, Sommariva M et al (2018) Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res 25(3):989–999
Roland CL, Lynn KD, Toombs JE et al (2009) Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One 4(11):e7669
Acknowledgements
No specific funding, financial disclosures or assistance declared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ishii, T., Kawazoe, A. & Shitara, K. Dawn of precision medicine on gastric cancer. Int J Clin Oncol 24, 779–788 (2019). https://doi.org/10.1007/s10147-019-01441-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01441-x