Abstract
Objective
To evaluate the diagnostic accuracy of high-resolution T2w intraoperative magnetic resonance imaging (iMRI) for detecting pituitary adenoma remnants compared to contrast-enhanced T1-weighted images.
Methods
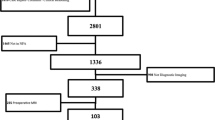
42 patients underwent iMRI-guided resection of large pituitary macroadenomas and fulfilled the inclusion criteria for this retrospective analysis. Intraoperative and postoperative imaging evaluation of tumor residuals and localization were assessed by two experienced neuroradiologists in a blinded fashion. The diagnostic accuracy of T2w and contrast-enhanced T1w images were evaluated.
Results
The diagnostic accuracy for detecting tumor residuals of high-resolution T2w images showed highly significant association to contrast-enhanced T1w images (p < 0.0001). Furthermore, identification rate of tumor remnants in different compartments, e.g., cavernous sinus, was comparable. In total, coronal T2w images provided a diagnostic sensitivity of 97.7% and specificity of 100% compared to the gold standard of contrast-enhanced T1w images. The postoperatively expected extent of resection proved to be true in 97.6% according to MRI 3 months after resection.
Conclusions
High-resolution T2w intraoperative MR images provide excellent diagnostic accuracy for detecting tumor remnants in macroadenoma surgery with highly significant association compared to T1w images with gadolinium. The routine-use and need of gadolinium in these patients should be questioned critically in each case in the future.




Similar content being viewed by others
References
Black PM, Moriarty T, Alexander E 3rd, Stieg P, Woodard EJ, Gleason PL, Martin CH, Kikinis R, Schwartz RB, Jolesz FA (1997) Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery 41(4):831–842 discussion 842–835
Sutherland GR, Kaibara T, Louw D, Hoult DI, Tomanek B, Saunders J (1999) A mobile high-field magnetic resonance system for neurosurgery. Journal of neurosurgery 91(5):804–813. https://doi.org/10.3171/jns.1999.91.5.0804
Hall WA, Liu H, Martin AJ, Pozza CH, Maxwell RE, Truwit CL (2000) Safety, efficacy, and functionality of high-field strength interventional magnetic resonance imaging for neurosurgery. Neurosurgery 46(3):632–641 discussion 641–632
Hall WA, Kowalik K, Liu H, Truwit CL, Kucharezyk J (2003) Costs and benefits of intraoperative MR-guided brain tumor resection. Acta neurochirurgica Supplement 85:137–142
Nimsky C, Ganslandt O, Von Keller B, Romstock J, Fahlbusch R (2004) Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology 233(1):67–78. https://doi.org/10.1148/radiol.2331031352
Nimsky C, Ganslandt O, Fahlbusch R (2005) Comparing 0.2 tesla with 1.5 tesla intraoperative magnetic resonance imaging analysis of setup, workflow, and efficiency. Academic radiology 12(9):1065–1079. https://doi.org/10.1016/j.acra.2005.05.020
Schwartz RB, Hsu L, Wong TZ, Kacher DF, Zamani AA, Black PM, Alexander E 3rd, Stieg PE, Moriarty TM, Martin CA, Kikinis R, Jolesz FA (1999) Intraoperative MR imaging guidance for intracranial neurosurgery: experience with the first 200 cases. Radiology 211(2):477–488. https://doi.org/10.1148/radiology.211.2.r99ma26477
Fahlbusch R, Ganslandt O, Nimsky C (2000) Intraoperative imaging with open magnetic resonance imaging and neuronavigation. Child’s nervous system: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 16(10–11):829–831. https://doi.org/10.1007/s003810000344
Nimsky C, Keller BV, Ganslandt O, Fahlbusch R (2006) Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery 59(1):105–114. https://doi.org/10.1227/01.neu.0000243289.98791.05
Jones J, Ruge J (2007) Intraoperative magnetic resonance imaging in pituitary macroadenoma surgery: an assessment of visual outcome. Neurosurgical focus 23(5):E12. https://doi.org/10.3171/FOC-07/11/E12
Gerlach R, du Mesnil de Rochemont R, Gasser T, Marquardt G, Reusch J, Imoehl L, Seifert V (2008) Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery 63(2):272–284; discussion 284–275. https://doi.org/10.1227/01.NEU.0000312362.63693.78
Bellut D, Hlavica M, Muroi C, Woernle CM, Schmid C, Bernays RL (2012) Impact of intraoperative MRI-guided transsphenoidal surgery on endocrine function and hormone substitution therapy in patients with pituitary adenoma. Swiss medical weekly 142:w13699. https://doi.org/10.4414/smw.2012.13699
Buchfelder M, Schlaffer SM (2012) Intraoperative magnetic resonance imaging during surgery for pituitary adenomas: pros and cons. Endocrine 42(3):483–495. https://doi.org/10.1007/s12020-012-9752-6
Coburger J, Konig R, Seitz K, Bazner U, Wirtz CR, Hlavac M (2014) Determining the utility of intraoperative magnetic resonance imaging for transsphenoidal surgery: a retrospective study. J Neurosurg 120(2):346–356. https://doi.org/10.3171/2013.9.JNS122207
Dort JC, Sutherland GR (2001) Intraoperative magnetic resonance imaging for skull base surgery. The Laryngoscope 111(9):1570–1575. https://doi.org/10.1097/00005537-200109000-00014
Fahlbusch R, Keller B, Ganslandt O, Kreutzer J, Nimsky C (2005) Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur J Endocrinol 153(2):239–248. https://doi.org/10.1530/eje.1.01970
Netuka D, Masopust V, Belsan T, Kramar F, Benes V (2011) One year experience with 3.0 T intraoperative MRI in pituitary surgery. Acta Neurochirurgica Supplement 109:157–159. https://doi.org/10.1007/978-3-211-99651-5_24
Sylvester PT, Evans JA, Zipfel GJ, Chole RA, Uppaluri R, Haughey BH, Getz AE, Silverstein J, Rich KM, Kim AH, Dacey RG, Chicoine MR (2015) Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free survival for pituitary adenomas. Pituitary 18(1):72–85. https://doi.org/10.1007/s11102-014-0560-2
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276(1):228–232. https://doi.org/10.1148/radiol.2015142690
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–617; discussion 617–618. https://doi.org/10.1227/00006123-199310000-00008
Serra C, Staartjes VE, Maldaner N, Muscas G, Akeret K, Holzmann D, Soyka MB, Schmid C, Regli L (2018) Predicting extent of resection in transsphenoidal surgery for pituitary adenoma. Acta Neurochir (Wien) 160(11):2255–2262. https://doi.org/10.1007/s00701-018-3690-x
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Hlavac M, Konig R, Halatsch M, Wirtz CR (2012) Intraoperative magnetic resonance imaging. Fifteen years’ experience in the neurosurgical hybrid operating suite. Der Unfallchirurg 115(2):121–124. https://doi.org/10.1007/s00113-011-2122-7
Patel KS, Yao Y, Wang R, Carter BS, Chen CC (2016) Intraoperative magnetic resonance imaging assessment of non-functioning pituitary adenomas during transsphenoidal surgery. Pituitary 19(2):222–231. https://doi.org/10.1007/s11102-015-0679-9
Elmholdt TR, Jorgensen B, Ramsing M, Pedersen M, Olesen AB (2010) Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus 3(3):285–287. https://doi.org/10.1093/ndtplus/sfq028
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB, International Society for Magnetic Resonance in M (2017) Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 16(7):564–570. https://doi.org/10.1016/S1474-4422(17)30158-8
Roder C, Breitkopf M, Ms BS, Freitas Rda S, Dimostheni A, Ebinger M, Wolff M, Tatagiba M, Schuhmann MU (2016) Beneficial impact of high-field intraoperative magnetic resonance imaging on the efficacy of pediatric low-grade glioma surgery. Neurosurgical focus 40(3):E13. https://doi.org/10.3171/2015.11.FOCUS15530
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local institutional review board (Project 208/2019/BO2).
Informed consent
In accordance with local privacy protection laws (§13(1) LDSG-Anpassungsgesetz) and EU regulation 2016/679 Art. 5, 6, 9, and 89, informed consent was waived for this retrospective evaluation of clinical data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gohla, G., Bender, B., Tatagiba, M. et al. Identification of tumor residuals in pituitary adenoma surgery with intraoperative MRI: do we need gadolinium?. Neurosurg Rev 43, 1623–1629 (2020). https://doi.org/10.1007/s10143-019-01202-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-019-01202-4