Abstract
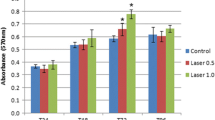
Photobiomodulation (PBM) has been shown to improve cell proliferation and cell migration. Many cell types have been investigated, with most studies using deep penetrating red light irradiation. Considering the interest of surface biostimulation of oral mesenchymal cells after surgical wound, the present study aimed to assess green light irradiation effects on Dental Pulp Stem Cells’ (DPSC) proliferation and migration. To understand the mechanisms underlying these effects, we investigated cytoskeleton organization and subsequent cell shape and stiffness. A 532-nm wavelength Nd:YAG laser (30 mW) was applied between 30 and 600 s on DPSC in vitro. Cell proliferation was analyzed at 24, 48, and 72 h after irradiation, by cell counting and enzymatic activity quantification (paranitrophenylphosphate phosphatase (pNPP) test). A wound healing assay was used to study cell migration after irradiation. Effects of PBM on cytoskeleton organization and cell shape were assessed by actin filaments staining. Elasticity changes after irradiation were quantified in terms of Young’s modulus measured using Atomic Force Microscopy (AFM) force spectroscopy. Green light significantly improved DPSC proliferation with a maximal effect obtained after 300-s irradiation (energy fluence 5 J/cm2). This irradiation had a significant impact on cell migration, improving wound healing after 24 h. These results were concomitant with a decrease of cells’ Young’s modulus after irradiation. This cell softening was explained by actin cytoskeleton reorganization, with diminution of cell circularity and more abundant pseudopodia. This study highlights the interest of green laser PMB for the proliferation and migration of mesenchymal stem cells, with encouraging results for clinical application, especially for surgical wound healing procedures.




Similar content being viewed by others
References
Brosseau L, Robinson V, Wells G, et al (2005) Low level laser therapy (classes I, II and III) for treating rheumatoid arthritis. Cochrane Database Syst Rev CD002049. https://doi.org/10.1002/14651858.CD002049.pub2
Yousefi-Nooraie R, Schonstein E, Heidari K, et al (2008) Low level laser therapy for nonspecific low-back pain. Cochrane Database Syst Rev CD005107. https://doi.org/10.1002/14651858.CD005107.pub4
Rankin IA, Sargeant H, Rehman H, Gurusamy KS (2017) Low-level laser therapy for carpal tunnel syndrome. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD012765
Chung H, Dai T, Sharma SK et al (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40:516–533. https://doi.org/10.1007/s10439-011-0454-7
Kneebone WJ (2006) Practical applications of low level laser therapy. Pract Pain Manag 6:34–40
Peplow PV, Chung T-Y, Ryan B, Baxter GD (2011) Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed Laser Surg 29:285–304. https://doi.org/10.1089/pho.2010.2846
Kushibiki T, Hirasawa T, Okawa S, Ishihara M (2015) Low reactive level laser therapy for mesenchymal stromal cells therapies. Stem Cells Int 2015:974864. https://doi.org/10.1155/2015/974864
Serrage H, Heiskanen V, Palin WM et al (2019) Under the spotlight: mechanisms of photobiomodulation concentrating on blue and green light. Photochem Photobiol Sci 18:1877–1909. https://doi.org/10.1039/c9pp00089e
Wang Y, Huang Y-Y, Wang Y et al (2016) Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep 6:33719. https://doi.org/10.1038/srep33719
de Eduardo FP, Bueno DF, de Freitas PM et al (2008) Stem cell proliferation under low intensity laser irradiation: a preliminary study. Lasers Surg Med 40:433–438. https://doi.org/10.1002/lsm.20646
Hadis MA, Cooper PR, Milward MR, et al (2015) The effect of UV-Vis to near-infrared light on the biological response of human dental pulp cells. In: Hamblin MR, Carroll JD, Arany P (eds). San Francisco, California, United States, p 930906
Holder MJ, Milward MR, Palin WM et al (2012) Effects of red light-emitting diode irradiation on dental pulp cells. J Dent Res 91:961–966. https://doi.org/10.1177/0022034512456040
Pereira LO, Longo JPF, Azevedo RB (2012) Laser irradiation did not increase the proliferation or the differentiation of stem cells from normal and inflamed dental pulp. Arch Oral Biol 57:1079–1085. https://doi.org/10.1016/j.archoralbio.2012.02.012
Marques MM, Diniz IMA, de Cara SPHM et al (2016) Photobiomodulation of dental derived Mesenchymal stem cells: a systematic review. Photomed Laser Surg 34:500–508. https://doi.org/10.1089/pho.2015.4038
Collart-Dutilleul P-Y, Chaubron F, De Vos J, Cuisinier FJ (2015) Allogenic banking of dental pulp stem cells for innovative therapeutics. World J Stem Cells 7:1010–1021. https://doi.org/10.4252/wjsc.v7.i7.1010
Luo Q, Kuang D, Zhang B, Song G (2016) Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim Biophys Acta 1860:1953–1960. https://doi.org/10.1016/j.bbagen.2016.06.010
Chen L, Jiang F, Qiao Y et al (2018) Nucleoskeletal stiffness regulates stem cell migration and differentiation through lamin A/C. J Cell Physiol 233:5112–5118. https://doi.org/10.1002/jcp.26336
Collart-Dutilleul P-Y, Secret E, Panayotov I et al (2014) Adhesion and proliferation of human mesenchymal stem cells from dental pulp on porous silicon scaffolds. ACS Appl Mater Interfaces 6:1719–1728. https://doi.org/10.1021/am4046316
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy 8:315–317. https://doi.org/10.1080/14653240600855905
Panayotov IV, Collart-Dutilleul P-Y, Salehi H, et al (2014) Sprayed cells and polyelectrolyte films for biomaterial functionalization: the influence of physical PLL-PGA film treatments on dental pulp cell behavior: Sprayed Cells and Polyelectrolyte Films for Biomaterial Functionalization …. Macromol Biosci 14:1771–1782. https://doi.org/10.1002/mabi.201400256
Liao X, Xie G-H, Liu H-W et al (2014) Helium-neon laser irradiation promotes the proliferation and migration of human epidermal stem cells in vitro: proposed mechanism for enhanced wound re-epithelialization. Photomed Laser Surg 32:219–225. https://doi.org/10.1089/pho.2013.3667
Sneddon IN (1965) The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int J Eng Sci 3:47–57. https://doi.org/10.1016/0020-7225(65)90019-4
Martin M, Benzina O, Szabo V et al (2013) Morphology and nanomechanics of sensory neurons growth cones following peripheral nerve injury. PLoS One 8:e56286. https://doi.org/10.1371/journal.pone.0056286
Collart-Dutilleul P-Y, Panayotov I, Secret E et al (2014) Initial stem cell adhesion on porous silicon surface: molecular architecture of actin cytoskeleton and filopodial growth. Nanoscale Res Lett 9:564. https://doi.org/10.1186/1556-276X-9-564
Krause M, Gautreau A (2014) Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol 15:577–590. https://doi.org/10.1038/nrm3861
Soares DM, Ginani F, Henriques ÁG, Barboza CAG (2015) Effects of laser therapy on the proliferation of human periodontal ligament stem cells. Lasers Med Sci 30:1171–1174. https://doi.org/10.1007/s10103-013-1436-9
Zaccara IM, Ginani F, Mota-Filho HG et al (2015) Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. Lasers Med Sci 30:2259–2264. https://doi.org/10.1007/s10103-015-1803-9
Carroll L, Humphreys TR (2006) LASER-tissue interactions. Clin Dermatol 24:2–7. https://doi.org/10.1016/j.clindermatol.2005.10.019
Liang C-C, Park AY, Guan J-L (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333. https://doi.org/10.1038/nprot.2007.30
Ayuk SM, Houreld NN, Abrahamse H (2018) Effect of 660 nm visible red light on cell proliferation and viability in diabetic models in vitro under stressed conditions. Lasers Med Sci 33:1085–1093. https://doi.org/10.1007/s10103-017-2432-2
Basso FG, Pansani TN, Cardoso LM et al (2018) Epithelial cell-enhanced metabolism by low-level laser therapy and epidermal growth factor. Lasers Med Sci 33:445–449. https://doi.org/10.1007/s10103-017-2176-z
Ejiri K, Aoki A, Yamaguchi Y et al (2014) High-frequency low-level diode laser irradiation promotes proliferation and migration of primary cultured human gingival epithelial cells. Lasers Med Sci 29:1339–1347. https://doi.org/10.1007/s10103-013-1292-7
Gagnon D, Gibson TWG, Singh A et al (2016) An in vitro method to test the safety and efficacy of low-level laser therapy (LLLT) in the healing of a canine skin model. BMC Vet Res 12. https://doi.org/10.1186/s12917-016-0689-5
Masson-Meyers DS, Bumah VV, Enwemeka CS (2016) Blue light does not impair wound healing in vitro. J Photochem Photobiol B Biol 160:53–60. https://doi.org/10.1016/j.jphotobiol.2016.04.007
Pansani TN, Basso FG, Turrioni APS et al (2017) Effects of low-level laser therapy and epidermal growth factor on the activities of gingival fibroblasts obtained from young or elderly individuals. Lasers Med Sci 32:45–52. https://doi.org/10.1007/s10103-016-2081-x
Pellicioli ACA, Martins MD, Dillenburg CS et al (2014) Laser phototherapy accelerates oral keratinocyte migration through the modulation of the mammalian target of rapamycin signaling pathway. J Biomed Opt 19:028002. https://doi.org/10.1117/1.JBO.19.2.028002
Rohringer S, Holnthoner W, Chaudary S et al (2017) The impact of wavelengths of LED light-therapy on endothelial cells. Sci Rep 7:10700. https://doi.org/10.1038/s41598-017-11061-y
Teuschl A, Balmayor ER, Redl H et al (2015) Phototherapy with LED light modulates healing processes in an in vitro scratch-wound model using 3 different cell types. Dermatol Surg 41:261–268. https://doi.org/10.1097/DSS.0000000000000266
Tsuka Y, Kunimatsu R, Gunji H et al (2019) Effects of Nd:YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: in vitro study. Lasers Med Sci 34:55–60. https://doi.org/10.1007/s10103-018-2586-6
Yin K, Zhu R, Wang S, Zhao RC (2017) Low-level laser effect on proliferation, migration, and antiapoptosis of mesenchymal stem cells. Stem Cells Dev 26:762–775. https://doi.org/10.1089/scd.2016.0332
Zou C, Luo Q, Qin J et al (2013) Osteopontin promotes mesenchymal stem cell migration and lessens cell stiffness via integrin β1, FAK, and ERK pathways. Cell Biochem Biophys 65:455–462. https://doi.org/10.1007/s12013-012-9449-8
Benzina O, Szabo V, Lucas O et al (2013) Changes induced by peripheral nerve injury in the morphology and nanomechanics of sensory neurons. J Biomed Opt 18:106014. https://doi.org/10.1117/1.JBO.18.10.106014
Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J (2014) Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 94:235–263. https://doi.org/10.1152/physrev.00018.2013
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Dental Pulp Stem Cells’ (DPSC) collection was declared to the Ministry of Higher Education, Research and Innovation: “Direction Générale de la Recherche et de l’Innovation,” Déclaration DC-2014-2198, Récépissé n°3119(bis). Clinical protocol for DPSC recovery was approved by the local ethical committee.
Informed consent
Written informed consents were obtained from the patients or the parents of the patients (for minor patients).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malthiery, E., Chouaib, B., Hernandez-Lopez, A.M. et al. Effects of green light photobiomodulation on Dental Pulp Stem Cells: enhanced proliferation and improved wound healing by cytoskeleton reorganization and cell softening. Lasers Med Sci 36, 437–445 (2021). https://doi.org/10.1007/s10103-020-03092-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03092-1