Abstract
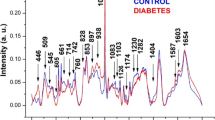
Raman spectroscopy has been employed in the quantitative analysis of biochemical components in human serum. This study aimed to develop a spectral model to estimate the concentration of glucose and lipid fractions in human serum, thus evaluating the feasibility of Raman spectroscopy technique for diagnostic purposes. A total of 44 samples of blood serum were collected from volunteers submitted to routine blood biochemical assay analysis. The biochemical concentrations of glucose, triglycerides, cholesterol, and high-density and low-density lipoproteins (HDL and LDL) were obtained by colorimetric method. Serum samples (200 μL) were submitted to Raman spectroscopy (830 nm, 250 mW, 50-s accumulation). The spectra of sera present peaks related to the main constituents, particularly proteins and lipids. A quantitative model based on partial least squares (PLS) regression has been developed to estimate the concentration of these compounds, taking the biochemical concentrations assayed by the colorimetric method as sample’s actual concentrations. The PLS model based on leave-one-out cross-validation approach estimated the concentration of triglycerides and cholesterol with r = 0.98 and 0.96, and root mean square error of 35.4 and 15.9 mg/dL, respectively. For the other biochemicals, the r was ranging from 0.75 to 0.86. These results evidenced the possibility of performing biochemical assay in blood serum samples by Raman spectroscopy and PLS regression and may be employed as a means of diagnosis in routine clinical analysis.




Similar content being viewed by others
References
Bachorik PS, Denke MA, Stein EA, Rifkind BM (2001) Lipids and dyslipoproteinemia. In: Henry JB (ed) Clinical diagnosis and management by laboratory methods, 20th edn. W. B. Saunders, Philadelphia, pp 224–248
Burtis CA, Ashwood ER, Bruns DE (2006) Tietz textbook of clinical chemistry, 4th edn. Elsevier Saunders, St. Louis, 2412 p
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106(25):3143–3421, http://www.nhlbi.nih.gov/sites/www.nhlbi.nih.gov/files/Circulation-2002-ATP-III-Final-Report-PDF-3143.pdf. Accessed 10 June 2016
Lam DW, LeRoith D (2015) Metabolic syndrome. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO (eds) Endotext. MDText.co, South Dartmouth, http://www.ncbi.nlm.nih.gov/books/NBK278936. Accessed 20 June 2016
Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Lloyd-Jones DM, Blum CB, McBride P, Eckel RH, Schwartz JS, Goldberg AC, Shero ST, Gordon D, Smith SC Jr, Levy D, Watson K, Wilson PW (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol 63(25 Pt B):2889–2934. doi:10.1016/j.jacc.2013.11.002
Van de Poll SWE, Römer TJ, Volger OL, Delsing DJM, Schut TCB, Princen HMG, Havekes LM, Jukema JW, van de Laarse A, Puppels GJ (2001) Raman spectroscopic evaluation of the effects of diet and lipid-lowering therapy on atherosclerotic plaque development in mice. Arterioscler Thromb Vasc Biol 21(10):1630–1635
Friedewald WT, Levy RI, Frederickson DS (1972) Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Bachorik PS, Ross JW (1995) National Cholesterol Education Program recommendations for the measurement of low-density lipoprotein cholesterol: executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin Chem 41(10):1414–1420
Jialal I (1996) A practical approach to the laboratory diagnosis of dyslipidemia. Am J Clin Pathol 106(1):128–138
Berger AJ, Itzkan I, Feld MS (1997) Feasibility of measuring blood glucose concentration by near-infrared Raman spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 53A(2):287–292
Rohleder D, Kocherscheidt G, Gerber K, Kiefer W, Köhler W, Möcks J, Petrich W (2005) Comparison of mid-infrared and Raman spectroscopy in the quantitative analysis of serum. J Biomed Opt 10(3):031108
Dingari NC, Barman I, Singh GP, Kang JW, Dasari RR, Feld MS (2011) Investigation of the specificity of Raman spectroscopy in non-invasive blood glucose measurements. Anal Bioanal Chem 400(9):2871–2880
Almeida ML, Saatkamp CJ, Fernandes AB, Pinheiro ALB, Silveira L (2016) Estimating the concentration of urea and creatinine in the human serum of normal and dialysis patients through Raman spectroscopy. Lasers Med Sci 7:1415–1423
Shao J, Lin M, Li Y, Li X, Liu J, Liang J, Yao H (2012) In vivo blood glucose quantification using Raman spectroscopy. PLoS One 7(10):e48127
Berger AJ, Koo T-W, Itzkan I, Horowitz G, Feld MS (1999) Multicomponent blood analysis by near-infrared Raman spectroscopy. Appl Opt 38(3):2916–2926
Qi D, Berger AJ (2007) Chemical concentration measurement in blood serum and urine samples using liquid-core optical fiber Raman spectroscopy. Appl Opt 46(10):1726–1734
Barman I, Narahara CD, Kang JW, Horowitz GL, Ramachandra RD, Feld MS (2012) Raman spectroscopy-based sensitive and specific detection of glycated hemoglobin. Anal Chem 84(5):2474–2482
Dingari NC, Horowitz GL, Kang JW, Dasari RR, Barman I (2012) Raman spectroscopy provides a powerful diagnostic tool for accurate determination of albumin glycation. PLoS One 7(2):e32406
Amer MS (2009) Raman spectroscopy for soft matter applications. Wiley, Hoboken, 301 p
Hanlon EB, Manoharan R, Koo T-W, Shafer KE, Motz JT, Fitzmaurice M, Kramer JR, Itzkan I, Dasari RR, Feld MS (2000) Prospects for in vivo Raman spectroscopy. Phys Med Biol 45(2):R1–R59
Manoharan R, Wang Y, Feld MS (1996) Histochemical analysis of biological tissues using Raman spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 52(2):215–249
Moreira LM, Silveira L, Santos FV, Lyon JP, Rocha R, Zângaro RA, Villaverde AB, Pacheco MTT (2008) Raman spectroscopy: a powerful technique for biochemical analysis and diagnosis. Spectroscopy Int J 22(1):1–19
McMurdy JW, Berger AJ (2003) Raman spectroscopy-based creatinine measurement in urine samples from a multipatient population. Appl Spectrosc 57(5):522–525
Dou X, Yamaguchi Y, Yamamoto H, Doi S, Ozaki Y (1996) Quantitative analysis of metabolites in urine using a highly precise, compact near-infrared Raman spectrometer. Vib Spectrosc 13(1):83–89
Bispo JAM, Vieira EES, Silveira L Jr, Fernandes AB (2013) Correlating the amount of urea, creatinine, and glucose in urine from patients with diabetes mellitus and hypertension with the risk of developing renal lesions by means of Raman spectroscopy and principal component analysis. J Biomed Opt 18(8):087004
Saatkamp CJ, Almeida ML, Bispo JAM, Pinheiro ALB, Fernandes AB, Silveira L (2016) Quantifying creatinine and urea in human urine through Raman spectroscopy aiming at diagnosis of kidney disease. J Biomed Opt 21(3):037001
Filho AC, Silveira L, Yanai AL, Fernandes AB (2015) Raman spectroscopy for a rapid diagnosis of sickle cell disease in human blood samples: a preliminary study. Lasers Med Sci 30(1):247–253
Pichardo-Molina JL, Frausto-Reyes C, Barbosa-García O, Huerta-Franco R, González-Trujillo JL, Ramírez-Alvarado CA, Gutiérrez-Juárez G, Medina-Gutiérrez C (2007) Raman spectroscopy and multivariate analysis of serum samples from breast cancer patients. Lasers Med Sci 22(4):229–236
González-Solís JL, Martínez-Espinosa JC, Torres-González LA, Aguilar-Lemarroy A, Jave-Suárez LF, Palomares-Anda P (2014) Cervical cancer detection based on serum sample Raman spectroscopy. Lasers Med Sci 29(3):979–985
Fukuyama N, Homma K, Wakana N, Kudo K, Suyama A, Ohazama H, Tsuji C, Ishiwata K, Eguchi Y, Nakazawa H, Tanaka E (2008) Validation of the Friedewald equation for evaluation of plasma LDL-Cholesterol. J Clin Biochem Nutr 843(1):1–5
Food and Drug Administration. Office of Regulatory Affairs (2015) Investigations operations manual. Appendix C. Food and Drug Administration, Silver Spring. http://www.fda.gov/downloads/ICECI/Inspections/IOM/UCM123517.pdf. Accessed 20 June 2016
Martens H, Naes T (1989) Multivariate calibration. Wiley, New York, 438 p
Beebe KR, Kowalski B (1987) An introduction to multivariate calibration and analysis. Anal Chem 59(17):1007–1017
Nunes CA, Freitas MP, Pinheiro ACM, Bastos SC (2012) Chemoface: a novel free user-friendly interface for chemometrics. J Braz Chem Soc 23(11):2003–2010
Movasaghi Z, Rehman S, Rehman I (2007) Raman spectroscopy of biological tissues. Appl Spectrosc Rev 42(5):493–541
Buschman HP, Deinum G, Motz JT, Fitzmaurice M, Kramer JR, van der Laarse A, Bruschke AV, Feld MS (2001) Raman microspectroscopy of human coronary atherosclerosis: biochemical assessment of cellular and extracellular morphologic structures in situ. Cardiovasc Pathol 10(2):69–82
Silveira L, Silveira FL, Bodanese B, Zângaro RA, Pacheco MTT (2012) Discriminating model for diagnosis of basal cell carcinoma and melanoma in vitro based on the Raman spectra of selected biochemicals. J Biomed Opt 17(7):077003
Ibrahim M, Alaam M, El-Haes H, Jalbout AF, de Leon A (2006) Analysis of the structure and vibrational spectra of glucose and fructose. Ecletica Quim 31(3):15–21
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F (2005) Diagnosis and management of the metabolic syndrome. An American heart association/national heart, lung, and blood institute scientific statement. Circulation 112(17):2735–2752. doi:10.1161/CIRCULATIONAHA.105.169404
Borges RCF, Navarro RS, Giana HE, Tavares FG, Fernandes AB, Silveira L (2015) Detecting alterations of glucose and lipid components in human serum by near-infrared Raman spectroscopy. Res Biomed Eng 31(2):160–168
Weatherby D, Ferguson S (2004) Blood chemistry and CBC analysis: clinical laboratory testing from a functional perspective. Emperors Group, Ashland
Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS (2011) The human serum metabolome. PLoS One 6(2):e16957
Acknowledgements
L Silveira Jr. thanks FAPESP (São Paulo Research Foundation, Brazil) for the financial support to obtain the Raman spectrometer (Grant no. 2009/01788-5). Authors thank Fernanda Grubisich Tavares from Laboratório Oswaldo Cruz for preparing the serum samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Role of funding sources
The project has been supported in part by a public funding agency (São Paulo Research Foundation—FAPESP), Process FAPESP no. 2009/01788-5, that allowed to purchase the Raman spectrometer.
Laboratório Oswaldo Cruz, owned by one of the authors (Hector Enrique Giana), has provided the serum samples and performed the biochemical assay used as the gold standard.
Ethical approval
The project has been submitted and received approval from the Research Ethics Committee of the Universidade Camilo Castelo Branco (Process no. 315993, CAAE no. 16464913400005494).
Informed consent
Since we used human serum samples from routine laboratory assay, the laboratory provided the informed consent.
Rights and permissions
About this article
Cite this article
Silveira, L., Borges, R.d.F., Navarro, R.S. et al. Quantifying glucose and lipid components in human serum by Raman spectroscopy and multivariate statistics. Lasers Med Sci 32, 787–795 (2017). https://doi.org/10.1007/s10103-017-2173-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2173-2