Abstract
Background
Inherited nemaline myopathy is one of the most common congenital myopathies. This genetically heterogeneous disease is defined by the presence of nemaline bodies in muscle biopsy. The phenotypic spectrum is wide and cognitive involvement has been reported, although not extensively evaluated.
Methods
We report two nemaline myopathy patients presenting pronounced central nervous system involvement leading to functional compromise and novel facial and skeletal dysmorphic findings, possibly expanding the disease phenotype.
Results
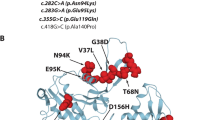
One patient had two likely pathogenic NEB variants, c.2943G > A and c.8889 + 1G > A, and presented cognitive impairment and dysmorphic features, and the other had one pathogenic variant in ACTA1, c.169G > C (p.Gly57Arg), presenting autism spectrum disorder and corpus callosum atrophy. Both patients had severe cognitive involvement despite milder motor dysfunction.
Conclusion
We raise the need for further studies regarding the role of thin filament proteins in the central nervous system and for a systematic cognitive assessment of congenital myopathy patients.


Similar content being viewed by others
Data availability
All available data was included in the manuscript and associated tables and figures.
References
Wallgren-Pettersson C, Sewry CA, Nowak KJ, Laing NG (2011) Nemaline myopathies. Seminars Paediatr Neurol 18(4):230–238. https://doi.org/10.1016/j.spen.2011.10.004
Amburgey K, Acker M, Saeed S, Amin R, Beggs AH, Bönnemann CG et al (2021) A cross-sectional study of nemaline myopathy. Neurol 96(10):e1425–e1436. https://doi.org/10.1212/WNL.0000000000011458
Moreno CAM, Abath Neto O, Donkervoort S, Hu Y, Reed UC, Oliveira ASB et al (2017) Clinical and histologic findings in ACTA1-related nemaline myopathy: case series and review of the literature. Pediatr Neurol 75:11–16. https://doi.org/10.1016/j.pediatrneurol.2017.04.002
Ryan MM, Schnell C, Strickland CD, Shield LK, Morgan G, Iannaccone ST et al (2001) Nemaline myopathy: a clinical study of 143 cases. Ann Neurol 50(3):312–320. https://doi.org/10.1002/ana.1080
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38(16):e164–e164. https://doi.org/10.1093/nar/gkq603
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A et al (2016) The ensemble variant effect predictor. Genome Biol 17(122):1–14. https://doi.org/10.1186/s13059-016-0974-4
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I et al (2005) The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Pike NA, Poulsen MK, Woo MA (2017) Validity of the Montreal cognitive assessment screener in adolescents and young adults with and without congenital heart disease. Nurs Res 66(3):222–230. https://doi.org/10.1097/NNR.0000000000000192
Schopler E, Reichler RJ, DeVellis RF, Daly K (1980) Toward objective classification of childhood autism: childhood autism rating scale (CARS). J Autism Dev Disord 10(1):91–103. https://doi.org/10.1007/BF02408436
Lehtokari VL, Kiiski K, Sandaradura SA, Laporte J, Repo P, Frey JA et al (2014) Mutation update: the spectra of nebulin variants and associated myopathies. Hum Mutat 35(12):1418–1926. https://doi.org/10.1002/humu.22693
Banihani R, Smile S, Yoon G, Dupuis A, Mosleh M, Snider A et al (2015) Cognitive and neurobehavioral profile in boys with duchenne muscular dystrophy. J Child Neurol 30(11):1472–1482. https://doi.org/10.1177/0883073815570154
Douniol M, Jacquette A, Cohen D, Bodeau N, Rachidi L, Angeard N et al (2012) Psychiatric and cognitive phenotype of childhood myotonic dystrophy type 1. Dev Med Child Neurol 54(10):905–911. https://doi.org/10.1111/j.1469-8749.2012.04379.x
Brun BN, Mockler SRH, Laubscher KM, Stephan CM, Wallace AM, Collison JA, et al (2017) Comparison of brain MRI findings with language and motor function in the dystroglycanopathies. Neurology 88(7):623–629. https://doi.org/10.1212/WNL.0000000000003609
Specht S, Straub V (2021) Intellectual disability in paediatric patients with genetic muscle diseases. Neuromuscul Disord 31(10):988–997. https://doi.org/10.1016/j.nmd.2021.08.012
Wallgren-Pettersson C (1989) Congenital nemaline myopathy: a clinical follow-up of twelve patients. J Neurol Sci 89(1):1–14. https://doi.org/10.1016/0022-510x(89)90002-6
Moreno CAM, Artilheiro MC, Fonseca ATQSM, Camelo CG, de Medeiros GC, Sassi FC et al (2023) Clinical manifestation of nebulin-associated nemaline myopathy. Neurol Genet 9(1):1–10. https://doi.org/10.1212/NXG.0000000000200056
Saito Y, Komaki H, Hattori A, Takeuchi F, Sasaki M, Kawabata K et al (2011) Extramuscular manifestations in children with severe congenital myopathy due to ACTA1 gene mutations. Neuromuscul Disord 21(7):489–493. https://doi.org/10.1016/j.nmd.2011.03.004
Shmueli O, Horn-Saban S, Chalifa-Caspi V, Shmoish M, Ophir R, Benjamin-Rodrig H et al (2003) GeneNote: whole genome expression profiles in normal human tissues C R Biol 326(10,11):1067–1072. https://doi.org/10.1016/j.crvi.2003.09.012
Kalajzic I, Kalajzic Z, Wang L, Jiang X, Lamothe K, San Miguel SM et al (2007) Pericyte/myoblast phenotype of osteoprogenitor cell. J Musculoskelet Neuronal Interact 7(4):320–322
Chandra M, Mamidi R, Ford S, Hidalgo C, Witt C, Ottenheijm C et al (2009) Nebulin alters cross-bridge cycling kinetics and increases thin filament activation: a novel mechanism for increasing tension and reducing tension cost. J Biol Chem 284(45):30889–30896. https://doi.org/10.1074/jbc.M109.049718
Laitila J, Hanif M, Paetau A, Hujanen S, Keto J, Somervuo P et al (2012) Expression of multiple nebulin isoforms in human skeletal muscle and brain. Muscle Nerve 46(5):730–777
Blokhuis AM, Deenen JCW, Voermans NC, van Engelen BGM, Kievit W, Groothuis JT (2021) The socioeconomic burden of facioscapulohumeral muscular dystrophy. J Neurol 268(12):4778–4788. https://doi.org/10.1007/s00415-021-10591-w
Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB et al (2014) Most genetic risk for autism resides with common variation. Nat Genet 46(8):881–885. https://doi.org/10.1038/ng.3039
Astrea G, Battini R, Lenzi S, Frosini S, Bonetti S, Moretti E et al (2016) Learning disabilities in neuromuscular disorders: a springboard for adult life. Acta Myol 35(2):90–95
D’Angelo MG, Bresolin N (2006) Cognitive impairment in neuromuscular disorders. Muscle Nerve 34(1):16–33. https://doi.org/10.1002/mus.20535
Acknowledgements
This study received funding from the Brazilian National Council for Scientific and Technological Development (CNPq) through the author Pedro Braga Neto as research grant funding (productivity scholarship).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The research has been approved by the local ethics committee under the number 5.952.628.
Consent for publication
The patients have signed an informed consent form for publication of clinical data.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nóbrega, P.R., de Brito de Souza, J.L., Maurício, R.B. et al. Marked neuropsychiatric involvement and dysmorphic features in nemaline myopathy. Neurol Sci 45, 1225–1231 (2024). https://doi.org/10.1007/s10072-023-07128-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07128-6