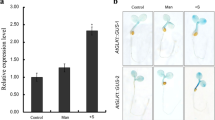
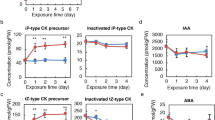
Abstract
In higher plants, the regulation of anthocyanin synthesis by various factors including light, sugars and hormones is mediated by numerous regulatory factors acting at the transcriptional level. Here, the association between sucrose and the plant hormone, cytokinin, in the presence of light was investigated to elucidate cytokinin signaling cascades leading to the transcriptional activation of anthocyanin biosynthesis genes in Arabidopsis seedlings. We showed that cytokinin enhances anthocyanin content and transcript levels of sugar inducible structural gene UDPglucose: flavonoid 3-O-glucosyl transferase (UF3GT) and regulatory gene PRODUCTION OF ANTHOCYANIN PIGMENT 1 (PAP1). Genetic analysis showed that cytokinin signaling modulates sugar-induced anthocyanin biosynthesis through a two-component signaling cascade involving the type-B response regulators ARR1, ARR10 and ARR12 in a redundant manner. Genetic, physiological and molecular biological approaches demonstrated that cytokinin enhancement is partially dependent on phytochrome and cryptochrome downstream component HY5, but mainly on photosynthetic electron transport. Taken together, we suggest that cytokinin acts down-stream of the photosynthetic electron transport chain in which the plastoquinone redox poise is modulated by sugars in a photoreceptor independent manner.
Similar content being viewed by others
References
Anantharaman, V., and Aravind, L. (2001). The CHASE domain: a predicted ligand-binding module in plant cytokinin receptors and other eukaryotic and bacterial receptors. Trends Biochem. Sci. 26, 579–582.
Argyros, R.D., Mathews, D.E., Chiang, Y.H., Palmer, C.M., Thibault, D.M., Etheridge, N., Argyros, D.A., Mason, M.G., Kieber, J.J., and Schaller, G.E. (2008). Type-B response regulators of Arabidopsis play key roles in cytokinin signalling and plant development. Plant Cell 20, 2102–2116.
Bellafiore, S., Barneche, F., Peltier, G., and Rochaix, J.D. (2005). State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895.
Chini, A., Fonseca, S., Fernandez, G., Adie, B., Chico, J.M., Lorenzo, O., Garcia-Casado, G., Lopez-Vidriero, I., Lozano, F.M., Ponce, M.R., et al. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666–671.
Das, P.K., Bang, G., Choi, S.B., Yoo, S.D., and Park Y.-I. (2010). Photosynthesis-dependent anthocyanin pigmentation in Arabidopsis. Plant Signal. Behav. 6, 1–4.
Gonzalez, A., Zhao, M., Leavitt, J.M., and Lloyd, A.M. (2008). Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 53, 814–827.
Guo, J.C., Hu, X.W., and Duan, R.J. (2005). Interactive effects of cytokinins, light and sucrose on the phenotypes and the syntheses of anthocyanins, lignins in cytokinin over-producing transgenic Arabidopsis. J. Plant Growth Regul. 24, 93–101.
Jeong, S.W., Das, P.K., Jeoung, S.C., Song, J.Y., Lee, H.K., Kim, Y.K., Kim, W.J., Park, Y.I., Yoo, S.D., Choi, S.B., et al. (2010). Ethylene suppression of sugar-Induced anthocyanin pigmentation in Arabidopsis thaliana. Plant Physiol. 154, 1514–1531.
Jung, C., Shim, J.S., Seo, J.S., Lee, H.Y., Kim, C.H., Choi, Y.D., and Cheong, J.-J. (2010). Non-specific phytohormonal induction of AtMYB44 and suppression of jasmonate-responsive gene activation in Arabidopsis thaliana. Mol. Cells 29, 71–76.
Kim, J.S., Lee, B.H., Kim, S.H., Ok, K.H., and Cho, K.Y. (2006). Response to environmental and chemical signals for anthocyanin biosynthesis in non-chlorophyllous corn (Zea mays L.) leaf. J. Plant Biol. 49, 16–25.
Lau, O.S., and Deng, X.Y. (2010). Plant hormone signaling lightens up: integrators of light and hormones. Curr. Opin. Plant Biol. 13, 571–577.
Loreti, E., Povero, G., Novi, G., Solfanelli, C., Alpi, A., and Perat, P. (2008). Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 179, 1004–1016.
Mason, M.G., Mathews, D.E., Argyros, D.A., Maxwell, B.B., Kieber, J.J., Alonso, J.M., Ecker, J.R., and Schaller, G.E. (2005). Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 17, 3007–3018.
Mehrtens, F., Kranz, H., Bednarek, P., and Weisshaar, B. (2005). The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 138, 1083–1096.
Moore, B., Zhou, L., Rolland, F., Hall, Q., Cheng, W.H., Liu, Y.X., Hwang, I., Jones, T., and Sheen, J. (2003). Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300, 332–336.
Nesi, N., Debeaujon, I., Jond, C., Pelletier, G., Caboche, M., and Lepiniec, L. (2000). The TT8 gene encodes a basic helix-loophelix domain protein required for expression of DFR and BAN genes in arabidopsis siliques. Plant Cell 12, 1863–1878.
Nesi, N., Jond, C., Debeaujon, I., Caboche, M., and Lepiniec, L. (2001). The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13, 2099–2114.
Page, M., Sultana, N., Paszkiewicz, K., Florance, H., and Smirnoff, N. (2011). The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 35, 388–404.
Peng, Z., Han, C., Yuan, L., Zhang, K., Huang, H., and Ren, C. (2011). Brassinosteroid enhances jasmonate-induced anthocyanin accumulation in Arabidopsis Seedlings. J. Integr. Plant Biol. 53, 632–640.
Qi, T., Song, S., Ren, Q., Wu, D., Huang, H., Chen, Y., Fan, M., Peng, W., Ren. C., and Xie, D. (2011). The jasmonate-ZIM-domain proteins interact with the WD-repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23, 1795–1814.
Rabino, I., and Mancinelli, A.L. (1986). Light temperature and anthocyanin production. Plant Physiol. 81, 922–924.
Riefler, M., Novak, O., Strnad, M., and Schmülling, T. (2006). Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development and cytokinin metabolism. Plant Cell 18, 40–54.
Roe, J.H. (1934). A colorimetric method for the determination of fructose in urine. J. Biol. Chem. 107, 15–22.
Schmülling, T. (2004). Cytokinin. In Encyclopedia of Biological Chemistry, W. Lennarz and M.D. Lane, eds. (Academic Press/Elsevier Science), pp. 1–7.
Schneider, M.J., and Stimson, W.R. (1971). Contribution of photosynthesis and phytochrome to the formation of anthocyanin in turnip seedlings. Plant Physiol. 48, 312–315.
Shan, X., Zhang, Y., Peng, W., Wang, Z., and Xie, D. (2009). Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 13, 3849–3860.
Shin, J., Park, E., and Choi, G. (2007). PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 49, 981–994.
Sivitz, A.B., Reinders, A., and Ward, J.M. (2008). Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 147, 92–100.
Solfanelli, C., Poggi, A., Loreti, E., Alpi, A., and Perata, P. (2006). Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 140, 637–646.
Stracke, R., Ishihara, H., Huep, G., Barsch, A., Mehrtens, F., Niehaus, K., and Weisshaar, B. (2007). Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 50, 660–677.
Teng, S., Keurentjes, J., Bentsink, L., Koornneef, M., and Smeekens, S. (2005). Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 139, 1840–1852.
Thines, B., Katsir, L., Melotto, M., Niu, Y., Mandaokar, A., Liu, G., Nomura, K., He, S.Y., Howe, G.A., and Browse, J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signaling. Nature 448, 661–665.
To, J.P., Haberer, G., Ferreira, F.J., Deuere, J., Mason, M.G., Schaller, G.E., Alonso, J.M., Ecker, J.R., and Kieber, J.J. (2004). Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16, 658–671.
Trebst, A. (1980). Inhibitors in electron flow. Methods Enzymol. 69, 675–715.
Vainonen, J.P., Hansson, M., and Vener, A.V. (2005). STN8 protein kinase in Arabidopsis thaliana is specific in phosphorylation of photosystem II core proteins. J. Biol. Chem. 280, 33679–33686.
Vandenbussche, F., Habricot, Y., Condiff, A.S., Maldiney, R., Van der Straeten, D., and Ahmad, M. (2007). HY5 is a point of convergence between cryptochrome and cytokinin signalling pathways in Arabidopsis thaliana. Plant J. 49, 428–441.
Wade, H.K., Sohal, A.K., and Jenkins, G.I. (2003). Arabidopsis ICX1 is a negative regulator of several pathways regulating flavonoid biosynthesis genes. Plant Physiol. 131, 707–715.
Werner, T., Motyka, V., Laucou, V., Smets, R., Van Onckelen, H., and Schmulling, T. (2003). Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15, 2532–2550.
Xiao, W.Y., Sheen, J., and Jang, J.C. (2000). The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol. Biol. 44, 451–461.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
About this article
Cite this article
Das, P.K., Shin, D.H., Choi, SB. et al. Cytokinins enhance sugar-induced anthocyanin biosynthesis in Arabidopsis. Mol Cells 34, 93–101 (2012). https://doi.org/10.1007/s10059-012-0114-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-012-0114-2