Abstract
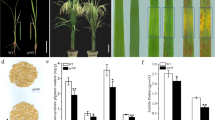
Spontaneous necrotic lesions were found in a lesion mimic mutant brown leaf spot 2 (bl2) without pathogenic infection. Small spots in the seedlings appeared at the four leaves stage and gradually grew into a large round and black area with a gray center on the leaf surfaces. Lower growth habit and lower agronomic trait values with reduced stature, tiller, and panicle number, as well as lower yield potential were noted in the mutants relative to the trait values of the wild-type plants. Microscopic analysis revealed that mesophyll chloroplast was severely damaged or absent in the spotted area of the mutant leaves. Total chlorophyll content, hydrogen peroxide level, and catalase activity were increased at up to 45 days after germination and were dropped at 60 d in the mutant leaves. However, the total protein contents were reduced slightly with a growth period of up to 45 days and were increased at 60 days after germination. A gradual increment of the total ascorbic acid contents in the mutants were observed with advanced plant age, but increased until 45 days and dropped comparatively at 60 days in the wild-type leaves. Increased gene transcriptions of OsPDI and OsGPX1 were noted in the spotted leaves as compared to the non-spotted leaves of the mutant and wild-type leaves, whereas transcripts of OsTPX were transcribed at lower levels in the spotted leaves as compared to the non-spotted leaves. The genetic nature of the bl2 mutant indicated that the F1 plants evidenced the wild-type phenotype and that bl2 was governed by a single recessive gene.
Similar content being viewed by others
References
Aebi, H.E. (1983). Catalase. In: Methods of Enzymatic Analysis. H.U. Bergmeyer, ed., (Weinhim, Germany: Verlag), pp. 273–286.
Ali, M.B., Hahn, E.J., and Paek, K.Y. (2005). Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. Environ. Exp. Bot. 54, 109–120.
Asada, K. (1999). The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639.
Badejo, A.A., Fujikawa, Y., and Esaka, M. (2009). Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff-Wheeler pathway in acerola (Malpighia glabra). J. Plant Physiol. 166, 652–660.
Baker, N.R. (1994). Chilling stress and photo synthesis. In: Causes of photo oxidative stress and amelioration of defense systems in plants. C.H. Foyer, and P.M. Mullineaux, eds., (Boca Raton, FL: USA, CRC Press), pp. 127–154.
Balague, C., Lin, B., Alcon, C., Flottes, G., Malmstrom, S., Köhler, C., Neuhaus, G., Pelletier, G., Gaymard, F., and Roby, D. (2003). HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 15, 365–79.
Bisht, S.S., Sharma, A., and Chaturvedi, K. (1989). Certain metabolic lesions of chromium toxicity in radish. Indian J. Agric. Biochem. 2, 109–115.
Bouchez, O., Huard, C., Lorrain, S., Roby, D., and Balagué, C. (2007). Ethylene is one of the key elements for cell death and defense response control in the Arabidopsis lesion mimic mutant vad. Plant Physiol. 145, 465–477.
Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Brennan, T., and Frenkel, C. (1977). Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 59, 411–416.
Brodersen, P., Petersen, M., Pike, H.M., Olszak, B., Skov, S., Odum, N., Jørgensen, L.B., Brown, R.E., and Mundy, J. (2002). Knockout of Arabidopsis ACCELERATED-CELLDEATH11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes Dev. 16, 490–502.
Büschges, R., Hollricher, K., Panstruga, R., Simons, G., Wolter, M., Frijters, A., van Daelen, R., van der Lee, T., Diergaarde, P., Groenendijk, J., et al. (1997). The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88, 695–705.
Chory, J., Peto, C.A., Ashbaugh, M., Saganich, R., Pratt, L., and Ausubel, F. (1989). Different roles for phytochrome in etiolated and green plants deduced from characterization of Arabidopsis thaliana mutants. Plant Cell 1, 867–880.
Conklin, P. (2001). Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ. 24, 383–394.
Dietrich, R.A., Delaney, T.P., Uknes, S.J., Ward, E.R., Ryals, J.A., and Dangl, J.L. (1994). Arabidopsis mutants simulating disease resistance response. Cell 77, 565–577.
Dietrich, R.A., Richberg, M.H., Schmidt, R., Dean, C., and Dangl, J.L. (1997). A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 88, 685–694.
Gray, J., Janick-Buckner, D., Buckner, B., Close, P.S., and Johal, G.S. (2002). Light-dependent death of maize IIs1 cells is mediated by mature chloroplasts. Plant Physiol. 130, 1894–1907.
Greenberg, J.T., Guo, A., Klessig, D.F., and Ausubel, F.M. (1994). Programmed cell death in plants: A pathogen triggered response activated coordinately with multiple defense functions. Cell 77, 551–563.
Hiraga, S., Sasaki, K., Ito, H., Ohashi, Y., and Matsui, H. (2001). A large family of class III plant peroxidases. Plant Cell Physiol. 42, 462–468.
Hoisington, D.A., Neuffer, M.G., and Walbot, V. (1982). Disease lesion mimics in maize. 1. Effect of genetic background, temperature, developmental age, and wounding on necrotic spot formation with Les1. Dev. Biol. 93, 381–388.
Hu, S.P., Zhou, Y., Zhang, L., Zhu, X.D., Li, L., Luo, L.J., Liu, G.L., and Zhou, Q.M. (2009). Correlation and quantitative trait loci analyses of total chlorophyll content and photosynthetic rate of rice (Oryza sativa) under water stress and well-watered conditions. J. Integr. Plant Biol. 9, 879–888.
Huang, L., Sun, Q., Qin, F., Li, C., Zhao, Y., and Zhou, D.X. (2007). Down-regulation of a SILENT INFORMATION REGULATOR2-related histone deacetylase gene, OsSRT1, induces DNA fragmentation and cell death in rice. Plant Physiol. 144, 1508–1519.
Ishikawa, A., Okamoto, H., Iwasaki, Y., and Asahi, T. (2001). A deficiency of coproporphyrinogen III oxidase causes lesion formation in Arabidopsis. Plant J. 27, 89–99.
Johal, G.S., Hulbert, S.H., and Briggs, S.P. (1995). Disease lesion mimics of maize: A model for cell death in plants. Bioessays 17, 685–692.
Kang, S.G., Jeong, H.K., and Suh, H.S. (2004). Characterization of a new member of the glutathione peroxidase gene family in Oryza sativa. Mol. Cells 17, 23–28.
Kang, S.G., Matin, M.N., Bae, H.H., and Natarajan, S. (2007). Proteome analysis and characterization of phenotypes of lesion mimic mutant spotted leaf 6 in rice. Proteomics 7, 2447–2458.
Kato, N., and Esaka, M. (1999). Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiol. Plant. 105, 321–329.
Kim, J.A., Cho, K., Singh, R., Jung, Y.H., Jeong, S.H., Kim, S.H., Lee, J.E., Cho, Y.S., Agrawal, G.K., Rakwal, R., et al. (2009). Rice OsACDR1 (Oryza sativa accelerated cell death and resistance 1) is a potential positive regulator of fungal disease resistance. Mol. Cells 28, 431–439.
Kinoshita, T. (1995). Report of committee on gene symbolization, nomenclature and linkage groups. Rice Genet. Newsl. 12, 9–115.
Lam, E., Kato, N., and Lawton, M. (2001). Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411, 848–853.
Liu, G., Wang, L., Zhou, Z., Leung, H., Wang, G.L., and He, C. (2004). Physical mapping of a rice lesion mimic gene, Spl1, to a 70-kb segment of rice chromosome 12. Mol. Gen. Genomics 272, 108–115.
Mackinney, G. (1941). Absorption of light by chlorophyll solutions. J. Biol. Chem. 140, 315–322.
Matin, M.N., Suh, H.S., and Kang, S.G. (2006). Characterization of phenotypes of rice lesion resembling disease mutants. Korean J. Genet. 28, 221–228.
Matin, M.N., Pandeya, D., Baek, K.H., Lee, D.S., Lee, J.H., Kang, H., and Kang, S.G. (2010). Phenotypic and genotypic analysis of rice lesion mimic mutants. Plant Pathol. J. 26, 159–169.
Mittler, R., Del Pozo, O., Meisel, L., and Lam, E. (1997). Pathogeninduced programmed cell death in plants, a possible defense mechanism. Dev. Genet. 21, 279–289.
Mizobuchi, R., Hirabayashi, H., Kaji, R., Nishizawa, Y., Yoshimura, A., Satoh, H., Ogawa, T., and Okamoto, M. (2002). Isolation and characterization of rice lesion-mimic mutants with enhanced resistance to rice blast and bacterial blight. Plant Sci. 163, 345–353.
Morel, J.B., and Dangl, J.L. (1997). The hypersensitive response and the induction of cell death in plants. Cell Death Differ. 4, 671–683.
Mori, M., Tomita, C., Sugimoto, K., Hasegawa, M., Hayashi, N., Dubouzet, J.G., Ochiai, H., Sekimoto, H., Hirochika, H., and Kikuchi, S. (2007). Isolation and molecular characterization of a Spotted leaf 18 mutant by modified activation-tagging in rice. Plant Mol. Biol. 63, 847–860.
Mosher, S., Moeder, W., Nishimura, N., Jikumaru, Y., Joo, S.H., Urquhart, W., Klessig, D.F., Kim, S.K., Nambara, E., and Yoshioka, K. (2010). The lesion-mimic mutant cpr22 shows alterations in abscisic acid signaling and abscisic acid insensitivity in a salicylic acid-dependent manner. Plant Physiol. 152, 1901–1913.
Nagato, Y., and Yoshimura, A. (1998). Report of the committee on gene symbolization nomenclature and linkage groups. Rice Genet. Newsl. 15, 13–74.
Noutoshi, Y., Kuromori, T., Wada, T., Hirayama, T., Kamiya, A., Imura, Y., Yasuda, M., Nakashita, H., Shirasu, K., and Shinozaki, K. (2006). Loss of necrotic spotted lesions 1 associates with cell death and defense responses in Arabidopsis thaliana. Plant Mol. Biol. 62, 29–42.
Pastori, G.M., Kiddle, G., Antoniw, J., Bernard, S., Veljovic-Jovanovic, S., Verrier, P.J., Noctor, G., and Foyer, C.H. (2003). Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15, 939–951.
Persson, M., Falk, A., and Dixelius, C. (2009). Studies on the mechanism of resistance to Bipolaris sorokiniana in the barley lesion mimic mutant bst1. Mol. Plant Pathol. 10, 587–598.
Qiao, Y., Jiang, W., Lee, J., Park, B., Choi, M.S., Piao, R., Woo, M.O., Roh, J.H., Han, L., Paek, N.C., et al. (2010). SPL28 encodes a clathrin-associated adaptor protein complex 1, medium subunit micro 1 (AP1M1) and is responsible for spotted leaf and early senescence in rice (Oryza sativa). New Phytol. 185, 258–274.
Ray, S., Anderson, J.M., Urmeev, F.I., and Goodwin, S.B. (2003). Rapid induction of a protein disulfide isomerase and defense-related genes in wheat in response to the hemibiotrophic fungal pathogen Mycosphaerella graminicola. Plant Mol. Biol. 53, 741–754.
Rostoks, N., Schmierer, D., Mudie, S., Drader, T., Brueggeman, R., Caldwell, D.G., Waugh, R., and Kleinhofs, A. (2006). Barley necrotic locus nec1 encodes the cyclic nucleotide-gated ion channel 4 homologous to the Arabidopsis HLM1. Mol. Genet. Genomics 275, 159–168.
Schröder, E., and Pointing, C.P. (1998). Evidence that peroxiredoxins are novel members of the thioredoxin fold superfamily. Protein Sci. 7, 2465–2468.
Simmons, C., Hantke, S., Grant, S., Johal, G.S., and Briggs, S.P. (1998). The maize lethal leaf spot 1 mutant has elevated resistant to fungal infection at the leaf epidermis. Mol. Plant Microbe Interact. 11, 1110–1118.
Singh, K., Multani, D.S., and Khush, G.S. (1995). A new spotted leaf mutant in rice. Rice Genet. Newsl. 12, 192–193.
Shin, S.Y., Kim, I.S., Kim, Y.H., Park, H.M., Lee, J.Y., Kang, H.G., and Yoon, H.S. (2008). Scavenging reactive oxygen species by rice dehydroascorbate reductase alleviates oxidative stresses in Escherichia coli. Mol. Cells 26, 616–620.
Smirnoff, N. (1996). The function and metabolism of ascorbic acid in plant. Ann. Bot. 78, 661–669.
Sugie, A., Murai, K., and Takumi, S. (2007). Alteration of respiration capacity and transcript accumulation level of alternative oxidase genes in necrosis lines of common wheat. Genes Genet. Syst. 82, 231–239.
Tokunaga, T., and Esaka, M. (2007). Induction of a novel XIP-type xylanase inhibitor by external ascorbic acid treatment and differential expression of XIP-family genes in rice. Plant Cell Physiol. 48, 700–714.
Tsunezuka, H., Fujiwara, M., Kawasaki, T., and Shimamoto, K. (2005). Proteome analysis of programmed cell death and defense signaling using the rice lesion mimic mutant cdr2. Mol. Plant-Microb Interact. 18, 52–59.
Walker, M.A., and McKersie, B.D. (1993). Role of the ascorbate-glutathione antioxidant system in chilling resistance of tomato. J. Plant Physiol. 141, 234–239.
Wang, F., Wang, G., Li, X., Huang, J., and Zheng, J. (2008). Heredity, physiology and mapping of a chlorophyll content gene of rice (Oryza sativa L.). J. Plant Physiol. 165, 324–330.
Wei, X., Xu, J., Guo, H., Jiang, L., Chen, S., Yu, C., Zhou, Z., Hu, P., Zhai, H., and Wan, J. (2010). DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol. 153, 1747–1758.
Wu, C., Bordeos, A., Madamba, M.R., Baraoidan, M., Ramos, M., Wang, G.L., Leach, J.E., and Leung, H. (2008). Rice lesion mimic mutants with enhanced resistance to diseases. Mol. Genet. Genomics 279, 605–619.
Yamanouchi, U., Yano, M., Lin, H., Ashikari, M., and Yamada, K. (2002). A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc. Natl. Acad. Sci. USA 99, 7530–7535.
Yin, Z., Chen, J., Zeng, L., Goh, M., Leung, H., Khush, G.S., and Wang, G.L. (2000). Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight. Mol. Plant-Microbe Interact. 13, 869–876.
Zeng, L.R., Qu, S., Bordeos, A., Yang, C., Baraoidan, M., Yan, H., and Xie, Q. (2004). Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-Box/Armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell 16, 2795–2808.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Matin, M.N., Saief, S.A., Rahman, M.M. et al. Comparative phenotypic and physiological characteristics of spotted Leaf 6 (spl6) and brown leaf Spot2 (bl2) Lesion Mimic Mutants (LMM) in rice. Mol Cells 30, 533–543 (2010). https://doi.org/10.1007/s10059-010-0151-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-010-0151-7