Abstract
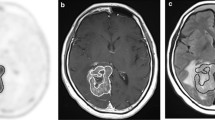
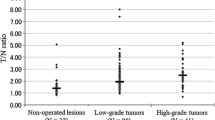
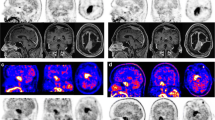
Anaplastic oligoastrocytoma (AOA) with necrosis is classified as glioblastoma (GBM) with oligodendroglioma component (GBMO), according to the 2007 World Health Organization classification. The prognosis of GBMO remains controversial because definitive diagnostic criteria regarding the percentage of the oligodendroglial components (OC) in the GBM do not exist. We previously reported dynamic methionine (MET) positron emission tomography (PET) in patients with these tumors. A significant decrease in the MET signal was seen in oligodendrocytic tumors, in contrast to a significant MET increase in GBMs. In this study, we analyzed the dynamic MET PET signal in four patients with primary (n = 2) and secondary (n = 2) GBMOs. Static PET scanning was performed in three consecutive phases. Both cases of primary GBMOs and one case of secondary GBMO presented with a gradual decrease in MET PET signal over the consecutive phases. In contrast, the remaining case of secondary GBMO presented with a pattern of slight increase. It is likely that the dynamic change of MET in patients with GBMO resemble those in patients with oligodendroglial tumor, however, further studies are needed to confirm them. We discuss the mechanisms from a viewpoint of pathological findings.




Similar content being viewed by others
References
Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK (1997) Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer 79:1381–1393
Decaestecker C, Camby I, Gordower L, Dewitte O, Cras P, Martin JJ, Pasteels JL, Van Ham P, Brotchi J, Kiss R, Salmon I (1998) Characterization of astroglial versus oligodendroglial phenotypes in glioblastomas by means of quantitative morphonuclear variables generated by computer-assisted microscopy. J Neuropathol Exp Neurol 57:791–802
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Wang Y, Li S, Chen L, You G, Bao Z, Yan W, Shi Z, Chen Y, Yao K, Zhang W, Kang C, Jiang T (2012) Glioblastoma with an oligodendroglioma component: distinct clinical behavior, genetic alterations, and outcome. Neuro Oncol 14:518–525
Klink B, Schlingelhof B, Klink M, Stout-Weider K, Patt S, Schrock E (2011) Glioblastomas with oligodendroglial component-common origin of the different histological parts and genetic subclassification. Cell Oncol (Dorr) 34:261–275
Ha SY, Kang SY, Do IG, Suh YL (2013) Glioblastoma with oligodendroglial component represents a subgroup of glioblastoma with high prevalence of IDH1 mutation and association with younger age. J Neurooncol 112:439–448
Hilton DA, Penney M, Pobereskin L, Sanders H, Love S (2004) Histological indicators of prognosis in glioblastomas: retinoblastoma protein expression and oligodendroglial differentiation indicate improved survival. Histopathology 44:555–560
Kraus JA, Lamszus K, Glesmann N, Beck M, Wolter M, Sabel M, Krex D, Klockgether T, Reifenberger G, Schlegel U (2001) Molecular genetic alterations in glioblastomas with oligodendroglial component. Acta Neuropathol 101:311–320
Salvati M, Formichella AI, D’Elia A, Brogna C, Frat A, Giangaspero F, Delfini R, Santoro A (2009) Cerebral glioblastoma with oligodendrogliomal component: analysis of 36 cases. J Neurooncol 94:129–134
Vordermark D, Ruprecht K, Rieckmann P, Roggendorf W, Vince GH, Warmouth-Metz M, Kolbl O, Flentje M (2006) Glioblastoma multiforme with oligodendroglial component (GBMO): favorable outcome after post-operative radiotherapy and chemotherapy with nimustine (ACNU) and teniposide (VM26). BMC Cancer 6:247
Appin CL, Gao J, Chisolm C, Torian M, Alexis D, Vincentelli C, Schniederjan MJ, Hadjipanayis C, Olson JJ, Hunter S, Hao C, Brat DJ (2013) Glioblastoma with oligodendroglioma component (GBM-O): molecular genetic and clinical characteristics. Brain Pathol 23:454–461
Miller CR, Dunham CP, Scheithauer BW, Perry A (2006) Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J Clin Oncol 24:5419–5426
Kanno H, Nishihara H, Narita T, Yamaguchi S, Kobayashi H, Tanino M, Kimura T, Terasaka S, Tanaka S (2012) Prognostic implication of histological oligodendroglial tumor component: clinicopathological analysis of 111 cases of malignant gliomas. PLoS One 7:7
He J, Mokhtari K, Sanson M, Marie Y, Kujas M, Huguet S, Leered P, Capelle L, Delattre JY, Poirier J, Hoang-Xuan K (2001) Glioblastomas with an oligodendroglial component: a pathological and molecular study. J Neuropathol Exp Neurol 60:863–871
Hegi ME, Janzer RC, Lambiv WL et al (2012) European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Presence of an oligodendroglioma-like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta Neuropathol 123:841–852
Homma T, Fukushima T, Vaccarella S, Yonekawa Y, Di Patre PL, Franceschi S, Ohgaki H (2006) Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropathol Exp Neurol 65:846–854
Nakamura H, Makino K, Kuratsu J (2011) Molecular and clinical analysis of glioblastoma with an oligodendroglial component (GBMO). Brain Tumor Pathol 28:185–190
Pinto LW, Araújo MB, Vettore AL, Wernersbach L, Leite AC, Chimelli LM, Soares FA (2008) Glioblastomas: correlation between oligodendroglial components, genetic abnormalities and prognosis. Virchows Arch 452:481–490
van den Bent MJ, Hegi ME, Stupp R (2006) Recent developments in the use of chemotherapy in brain tumours. Eur J Cancer 42:582–588
Jiang H, Ren X, Wang J, Zhang Z, Jia W, Lin S (2014) Short-term survivors in glioblastomas with oligodendroglioma component: a clinical study of 186 Chinese patients from a single institution. J Neurooncol 116:395–404
Aki T, Nakayama N, Yonezawa S, Takenaka S, Miwa K, Asano Y, Shinoda J, Yano H, Iwama T (2012) Evaluation of brain tumors using dynamic 11C-methionine-PET. J Neurooncol 109:115–122
Stupp R, Mason WP, van den Bent MJ, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Miyazaki M, Nishihara H, Terasaka S, Kobayashi H, Yamaguchi S, Ito T, Kamoshima Y, Fujimoto S, Kaneko S, Katoh M, Ishii N, Mohri H, Tanino M, Kimura T, Tanaka S (2014) Immunohistochemical evaluation of O6 -methylguanine DNA methyltransferase (MGMT) expression in 117 cases of glioblastoma. Neuropathology 34:268–276
Singh VY, Chacko G, Chacko AG, Rajshekhar V (2014) Fluorescence in situ hybridization for 1p, 19q status in a cohort of glial neoplasms. Neurol India 62:32–36
Giannini C, Burger PC, Berkey BA, Cairncross JG, Jenkins RB, Mehta M, Curran WJ, Aldape K (2008) Anaplastic oligodendroglial tumors: refining the correlation among histopathology, 1p 19q deletion and clinical outcome in Intergroup Radiation Therapy Oncology Group Trial 9402. Brain Pathol 18:360–369
McDonald JM, See SJ, Tremont IW, Colman H, Gilbert MR, Groves M, Burger PC, Louis DN, Giannini C, Fuller G, Passe S, Blair H, Jenkins RB, Yang H, Ledoux A, Aaron J, Tipnis U, Zhang W, Hess K, Aldape K (2005) The prognostic impact of histology and 1p/19q status in anaplastic oligodendroglial tumors. Cancer 104:1468–1477
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773
Myung JK, Cho HJ, Kim H, Park CK, Lee SH, Choi SH, Park P, Yoon JM, Park SH (2014) Prognosis of glioblastoma with oligodendroglioma component is associated with the IDH1 Mutation and MGMT methylation status. Transl Oncol 7:712–719
Figarella-Branger D, Mokhtari K, Colin C et al (2014) Prognostic relevance of histomolecular classification of diffuse adult high-grade gliomas with necrosis. Brain Pathol [Epub ahead of print]
Saito T, Maruyama T, Muragaki Y, Tanaka M, Nitta M, Shinoda J, Aki T, Iseki H, Kurisu K, Okada Y (2013) 11C-methionine uptake correlates with combined 1p and 19q loss of heterozygosity in oligodendroglial tumors. AJNR Am J Neuroradiol 34:85–91
Acknowledgments
This work was supported by a grant from MSD. The funders had no role in the study design, data collection and analysis, publication decision, or manuscript preparation.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yano, H., Ohe, N., Nakayama, N. et al. Dynamic study of methionine positron emission tomography in patients with glioblastoma with oligodendroglial components. Brain Tumor Pathol 32, 253–260 (2015). https://doi.org/10.1007/s10014-015-0218-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-015-0218-4