Abstract
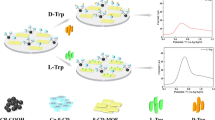
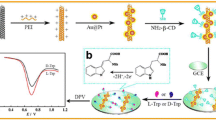
A novel and simple chiral sensing platform had been successfully fabricated by means of amidation reaction between 3, 4, 9, 10-perylenetetracarboxylic acid (PTCA) and chitosan (CS) to form 3, 4, 9, 10-perylenetetracarboxylic acid–chitosan (PTCA–CS) composite film. Since CS has chiral center and PTCA has excellent electrical conductivity, the PTCA–CS composite modified glassy carbon electrode (PTCA–CS/GCE) could be treated as an effective electrochemical chiral sensor and applied for chiral discrimination of tryptophan (Trp) enantiomers theoretically. PTCA–CS composite was characterized by Fourier transform infrared (FTIR) spectroscopy and cyclic voltammetry (CV). When the prepared chiral sensing interface interacted with tryptophan isomers, a higher selectivity was received from D-Trp by differential pulse voltammetry (DPV). It indicated that the PTCA–CS/GCE can be treated as an electrochemical chiral sensor for the discrimination of Trp enantiomers. Further study demonstrated that the peak currents were linearly increased with the increasing percentage of L-Trp of Trp racemic mixture. Furthermore, the enantioselective interaction of the PTCA–CS/GCE was systematically studied by other experimental factors, such as the incubation time and acidity.








Similar content being viewed by others
References
Miyazaki A, Nakamura T, Kawaradani M, Marumo S (1988) Resolution and biological activity of both enantiomers of methamidophos and acephate. J Agric Food Chem 36(4):835–837
Porpiglia N, Musile G, Bortolotti F, De Palo EF, Tagliaro F (2016) Chiral separation and determination of ketamine and norketamine in hair by capillary electrophoresis. Forensic Sci Int 266:304–310
Szabó ZI, Tóth G, Völgyi G, Komjáti B, Hancu G, Szente L, Noszál B (2016) Chiral separation of asenapine enantiomers by capillary electrophoresis and characterization of cyclodextrin complexes by NMR spectroscopy, mass spectrometry and molecular modeling. J Pharm Biomed 117:398–404
Grady T, Joyce T, Smyth MR, Diamond D, Harris SJ (1998) Chiral resolution of the enantiomers of phenylglycinol using (S)-di-naphthylprolinol calix [4] arene by capillary electrophoresis and fluorescence spectroscopy. Anal Commun 35(4):123–125
Kharaishvili Q, Jibuti G, Farkas T, Chankvetadze B (2016) Further proof to the utility of polysaccharide-based chiral selectors in combination with superficially porous silica particles as effective chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. J Chromatogr A 1467:163–168
Chennuru LN, Choppari T, Nandula RP, Zhang T, Franco P (2016) Direct separation of pregabalin enantiomers using a zwitterionic chiral selector by high performance liquid chromatography coupled to mass spectrometry and ultraviolet detection. Molecules 21:1578
Tang J, Pang L, Zhou J, Zhang S, Tang W (2016) Per (3-chloro-4-methyl) phenylcarbamate cyclodextrin clicked stationary phase for chiral separation in multiple modes high-performance liquid chromatography. Anal Chim Acta 946:96–103
Park GH, Lee YH, Chang SM, Kim WS, Kim JM (2016) Developing trends of the chiral drug separation and analysis technology by using molecular recognition. Clean Technol 22(2):75–81
Guo L, Huang Y, Zhang Q, Chen C, Guo D, Chen Y, Fu Y (2014) Electrochemical sensing for naproxen enantiomers using biofunctionalized reduced graphene oxide nanosheets. J Electrochem Soc 161(4):B70–B74
Bao L, Dai J, Yang L, Ma J, Tao Y, Deng L, Kong Y (2015) Electrochemical recognition of tyrosine enantiomers based on chiral ligand exchange with sodium alginate as the chiral selector. J Electrochem Soc 162(7):H486–H491
Zhang Q, Huang Y, Guo L, Chen C, Guo D, Chen Y, Fu Y (2014) DNA-based nanocomposite as electrochemical chiral sensing platform for the enantioselective interaction with quinine and quinidine. New J Chem 38(9):4600–4606
Kuhn A, Anson FC (1996) Effects of chirality during electrochemical oxidation of 2, 3 butanediol stereoisomers. J Electroanal Chem 410(2):243–246
Gao J, Njue CK, Mbindyo JK, Rusling JF (1999) Mechanism of stereoselective production of trans-1-decalone by electrochemical catalysis in microemulsions. J Electroanal Chem 464(1):31–38
Pleus S, Schulte B (2001) Poly (pyrroles) containing chiral side chains: effect of substituents on the chiral recognition in the doped as well as in the undoped state of the polymer film. J Solid State Electrochem 5(7-8):522–530
Gou H, He J, Mo Z, Wei X, Hu R, Wang Y, Guo R (2016) A highly effective electrochemical chiral sensor of tryptophan enantiomers based on covalently functionalize reduced graphene oxide with L-lysine. J Electrochem Soc 163(7):B272–B279
Doménech A, Alarcón J (2007) Microheterogeneous electrocatalytic chiral recognition at monoclinic vanadium-doped zirconias: enantioselective detection of glucose. Anal Chem 79(17):6742–6751
Chen Q, Zhou J, Han Q, Wang Y, Fu Y (2012) A new chiral electrochemical sensor for the enantioselective recognition of penicillamine enantiomers. J Solid State Electrochem 16(7):2481–2485
Wang Y, Han Q, Zhang Q, Huang Y, Guo L, Fu Y (2013) Enantioselective recognition of penicillamine enantiomers on bovine serum albumin-modified glassy carbon electrode. J Solid State Electrochem 17(3):627–633
Chen Y, Huang Y, Guo D, Chen C, Wang Q, Fu Y (2014) A chiral sensor for recognition of DOPA enantiomers based on immobilization of β-cyclodextrin onto the carbon nanotube-ionic liquid nanocomposite. J Solid State Electrochem 18(12):3463–3469
Yuan Y, Gou X, Yuan R, Chai Y, Zhuo Y, Ye X, Gan X (2011) Graphene-promoted 3, 4, 9, 10-perylenetetracarboxylic acid nanocomposite as redox probe in label-free electrochemical aptasensor. Biosens Bioelectron 30(1):123–127
Kampen T, Bekkali A, Thurzo I, Zahn DRT, Bolognesi A, Ziller T, Lugli P (2004) Barrier heights of organic modified Schottky contacts: theory and experiment. Appl Surf Sci 234(1-4):313–320
Vertsimakha Y, Lutsyk P, Palewska K, Sworakowski J, Lytvyn O (2007) Optical and photovoltaic properties of thin films of N, N′-dimethyl-3, 4, 9, 10-perylenetetracarboxylic acid diimide. Thin Solid Films 515(20-21):7950–7957
Huang Y, Guo D, Zhang Q, Guo L, Chen Y, Fu Y (2014) Chiral sensing for electrochemical impedance spectroscopy recognition of lysine enantiomers based on a nanostructured composite. RSC Adv 4(63):33457–33461
Guo D, Ran P, Chen C, Chen Y, Xuan C, Fu Y (2015) Study on chiral nanocomposite for recognition of DOPA enantiomers via square wave voltammetry. J Electrochem Soc 162(14):B354–B359
Özcan A, Şahin Y (2012) A novel approach for the selective determination of tryptophan in blood serum in the presence of tyrosine based on the electrochemical reduction of oxidation product of tryptophan formed in situ on graphite electrode. Biosens Bioelectron 31(1):26–31
Kim JH, Kim JH, Jegal J, Lee KH (2003) Optical resolution of α-amino acids through enantioselective polymeric membranes based on polysaccharides. J Membr Sci 213(1-2):273–283
Liu L, Li C, Bao C, Jia Q, Xiao P, Liu X, Zhang Q (2012) Preparation and characterization of chitosan/graphene oxide composites for the adsorption of Au (III) and Pd (II). Talanta 93:350–357
Niu X, Yang W, Ren J, Guo H, Long S, Chen J, Gao J (2012) Electrochemical behaviors and simultaneous determination of guanine and adenine based on graphene–ionic liquid–chitosan composite film modified glassy carbon electrode. Electrochim Acta 80:346–353
Weng X, Cao Q, Liang L, Chen J, You C, Ruan Y, Wu L (2013) Simultaneous determination of dopamine and uric acid using layer-by-layer graphene and chitosan assembled multilayer films. Talanta 117:359–365
Ou J, Tao Y, Xue J, Kong Y, Dai J, Deng L (2015) Electrochemical enantiorecognition of tryptophan enantiomers based on graphene quantum dots–chitosan composite film. Electrochem Commun 57:5–9
Wang M, Xiao FN, Wang K, Wang FB, Xia XH (2013) Electric field driven protonation/deprotonation of 3, 4, 9, 10-perylene tetracarboxylic acid immobilized on graphene sheets via π–π stacking. J Electroanal Chem 688:304–307
Tu FY, Yu LY, Yu JG, Chen XQ, Fu Q, Jiao FP, Zhang T (2013) Graphene as tunable stationary phase additive for enantioseparation. Nano 8(06):1350069
Berthod A (2006) Chiral recognition mechanisms. Anal Chem 78(7):2093–2099
Ou J, Zhu Y, Kong Y, Ma J (2015) Graphene quantum dots/β-cyclodextrin nanocomposites: a novel electrochemical chiral interface for tryptophan isomer recognition. Electrochem Commun 60:60–63
Yu Y, Liu W, Ma J, Tao Y, Qin Y, Kong Y (2016) An efficient chiral sensing platform based on graphene quantum dot–tartaric acid hybrids. RSC Adv 6(87):84127–84132
Chen Q, Zhou J, Han Q, Wang Y, Fu Y (2012) Electrochemical enantioselective recognition of tryptophane enantiomers based on chiral ligand exchange. Colloids Surf B 92:130–135
Zhang Q, Guo L, Huang Y, Wang Y, Han Q, Fu Y (2013) A reagentless enantioselective sensor for tryptophan enantiomers via nanohybrid matrices. Anal Methods 5(17):4397–4401
Xu J, Wang Q, Xuan C, Xia Q, Lin X, Fu Y (2016) Chiral recognition of tryptophan enantiomers based on β-cyclodextrin-platinum nanoparticles/graphene nanohybrids modified electrode. Electroanalysis 28(4):868–873
Funding
This work was supported by the National Natural Science Foundation of China (51262027), the financial support of the Natural Science Foundation of Gansu Province (1104GKCA019 and 1010RJZA023), the Science and Technology Tackle Key Problem Item of Gansu Province (2GS064-A52-036-08) and the fund of the State Key Laboratory of Solidification Processing in NWPU (SKLSP201011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mo, Z., Niu, X., Gao, H. et al. Electrochemical recognition for tryptophan enantiomers based on 3, 4, 9, 10-perylenetetracarboxylic acid–chitosan composite film. J Solid State Electrochem 22, 2405–2412 (2018). https://doi.org/10.1007/s10008-018-3960-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-3960-9