Abstract
Purpose
Lower lip squamous cell carcinomas (LLSCCs) exhibit lower levels of aggressiveness, low relations with metastases and better prognosis when compared with intraoral squamous cell carcinomas. Differently from the oral tongue squamous cell carcinomas (OTSCCs) have a high tendency towards local invasion and lymph nodal dissemination. Our aim was to evaluate tumor thickness in cases of oral squamous cell carcinoma and correlate it with histological grade of malignancy and GATA3 immunoreactivity.
Methods
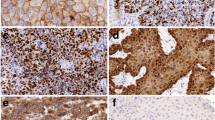
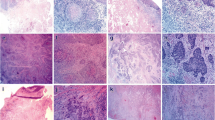
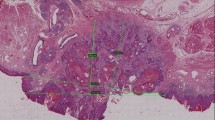
Sixty specimens (30 LLSCCs and 30 OTSCCs) were scanned and digitized for the subsequent measurement of tumor thickness, histopathological examination, and quantitative analysis of GATA3 in the parenchyma and stroma of the tumors.
Results
Tumor thickness was lower in LLSCC compared to OTSCCs. Immunohistochemical analysis of GATA3 in parenchyma, stroma and both compartments showed higher immunoreactivity in LLSCCs compared to OTSCCs. We observed a negative correlation between tumor thickness and GATA3 expression in parenchyma, stroma, and both compartments. Our results revealed the presence of GATA3 in all cases both in the parenchyma and in the stroma. Higher expression was more related to LLSCCs, which are known to be less aggressive tumors than OTSCCs.
Conclusions
A greater tumor thickness was found in OTSCCs, which was correlated with lower expression of GATA3, suggesting that this protein is involved in the inhibition of proliferative, migratory, and invasive capacity.



Similar content being viewed by others
Data availability
The data cannot be shared due to ethics.
References
Sarode G, Maniyar N, Sarode SC, Jafer M, Patil S, Awan KH (2020) Epidemiologic aspects of oral cancer. Dis Mon 66(12):100988. https://doi.org/10.1016/j.disamonth.2020.100988
Malkani N, Kazmi S, Rashid UM (2021) Epidemiological Assessment of oral Cancer Burden in Pakistan. Cancer Invest 39(10):842–853. https://doi.org/10.1080/07357907.2021.1982962
Roi A, Roi CI, Andreescu NI, Riviş M, Badea ID, Meszaros N et al (2020) Oral cancer histopathological subtypes in association with risk factors: a 5-year retrospective study. Rom J Morphol Embryol 61(4):1213–1220. https://doi.org/10.47162/RJME.61.4.22
Miranda-Filho A, Bray F (2020) Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol 102:104551. https://doi.org/10.1016/j.oraloncology.2019.104551
Freitas RSLS, Maia AC, Rodrigues RR, Rolim LSA, Souza LB, Andrade Santos PP (2021) Prevalence of lip and tongue squamous cell carcinoma (SCC) at a pathological anatomy service in Northeast Brazil. Int J Odontostomat 15(2):409–414
Wunschel M, Neumeier M, Utpatel K, Reichert TE, Ettl T, Spanier G (2021) Staging more important than grading? Evaluation of malignancy grading, depth of invasion, and resection margins in oral squamous cell carcinoma. Clin Oral Investig 25(3):1169–1182. https://doi.org/10.1007/s00784-020-03421-2
Almangush A, Mäkitie AA, Triantafyllou A, de Bree R, Strojan P, Rinaldo A et al (2020) Staging and grading of oral squamous cell carcinoma: an update. Oral Oncol 107:104799. https://doi.org/10.1016/j.oraloncology.2020.104799
Hu Y, Lv F, Li N, Yuan X, Zhang L, Zhao S et al (2023) Long noncoding RNA MEG3 inhibits oral squamous cell carcinoma progression via GATA3. FEBS Open Bio 13(1):195–208. https://doi.org/10.1002/2211-5463.13532
Zhang Q, Qi T, Long Y, Li X, Yao Y, Wu Q et al (2022) GATA3 predicts the Tumor Microenvironment phenotypes and molecular subtypes for bladder carcinoma. Front Surg 12:9:860663. https://doi.org/10.3389/fsurg.2022.860663
Hernández-Guerrero JC, Jacinto-Alemán LF, Jiménez-Farfán MD, Macario-Hernández A, Hernández-Flores F, Alcántara-Vázquez A (2013) Prevalence trends of oral squamous cell carcinoma. Mexico City’s General Hospital experience. Med Oral Patol Oral Cir Bucal 18(2):e306–311. https://doi.org/10.4317/medoral.18043
Almangush A, Bello IO, Keski-Säntti H, Mäkinen LK, Kauppila JH, Pukkila M et al (2014) Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck 36(6):811–818. https://doi.org/10.1002/hed.23380
Fang J, Li X, Ma D, Liu X, Chen Y, Wang Y et al (2017) Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer 17(1):375. https://doi.org/10.1186/s12885-017-3317-2
Ebrahimi A, Gil Z, Amit M, Yen TC, Liao CT, Chaturvedi P et al (2019) International Consortium for Outcome Research (ICOR) in Head and Neck Cancer. Depth of invasion alone as an indication for postoperative radiotherapy in small oral squamous cell carcinomas: an International Collaborative Study. Head Neck 41(6):1935–1942. https://doi.org/10.1002/hed.25633
Wetzel M, Strickley J, Haeberle MT, Brown TS (2019) Depth of Invasion of Aggressive and nonaggressive basal cell carcinoma. J Clin Aesthet Dermatol 12(3):12–14
Bai F, Zheng C, Liu X, Chan HL, Liu S, Ma J et al (2022) Loss of function of GATA3 induces basal-like mammary tumors. Theranostics 12(2):720–733. https://doi.org/10.7150/thno.65796
Mc Donald TM, Epstein JI (2021) Aberrant GATA3 staining in Prostatic Adenocarcinoma: a potential diagnostic pitfall. Am J Surg Pathol 45(3):341–346. https://doi.org/10.1097/PAS.0000000000001557
Zhang Z, Fang X, Xie G, Zhu J (2021) GATA3 is downregulated in HCC and accelerates HCC aggressiveness by transcriptionally inhibiting slug expression. Oncol Lett 21(3):231. https://doi.org/10.3892/ol.2021.12492
Medeiros Souza P, Carvalho FM, Aguiar FN, Gagliato D, Dornellas de Barros ACS (2022) Association between GATA3 and histopathological and immunohistochemical parameters in Early-Infiltrating Breast Carcinomas. Eur J Breast Health 18(3):229–234
Wang W, Wang M, Xu J, Long F, Zhan X (2020) Overexpressed GATA3 enhances the sensitivity of colorectal cancer cells to oxaliplatin through regulating MiR-29b. Cancer Cell Int 20:339. https://doi.org/10.1186/s12935-020-01424-3
El-Arabey AA, Denizli M, Kanlikilicer P, Bayraktar R, Ivan C, Rashed M et al (2020) GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell Signal 68:109539. https://doi.org/10.1016/j.cellsig.2020.109539
Chi Z, Balani J, Gopal P, Hammer S, Xu J, Peng L (2022) GATA3 positivity is associated with poor prognosis in patients with oesophageal squamous cell carcinoma. J Clin Pathol 17:208035. https://doi.org/10.1136/jclinpath-2021-208035
Guo W, Lee W, Lu Y, Li X, Chandan VS (2020) Incidence and significance of GATA3 positivity in gallbladder adenocarcinoma. Hum Pathol 106:39–44. https://doi.org/10.1016/j.humpath.2020.09.012
Sun J, Liu S, Fu K, Gao N, Li R, He W, Gao Z (2021) Clinicopathological characteristics and outcomes of 23 patients with secretory carcinoma of major salivary glands. Sci Rep 22(1):22639. https://doi.org/10.1038/s41598-021-01970-4
Funding
This study was funded by National Council for Scientific and Technological Development (CNPq) (Universal CNPq nº 424589/2016-8) - Pedro Paulo de Andrade Santos.
Author information
Authors and Affiliations
Contributions
P.P.A.S, A.G.B.V and M.C.M.C performed the experiments, C.R.M, A.G.B.V, P.P.A.S and M.C.M.C analysed the data, C.R.M and A.G.B.V prepared the figures, and P.P.A.S and C.R.M wrote the main manuscript text. M.C.M.C and A.G.B.V the experiments, P.P.A.S supervised the project, and L.B.S and H.C.G reviewed the manuscript text. P.P.A.S, L.B.S and H.C.G supervised the project, offered senior supervision. All authors reviewed the manuscript. Arthur Geovanni Borges Vital; Maria Carolina Magalhães de Carvalho; Caio Rodrigues Maia; Hébel Cavalcanti Galvão; Lélia Batista de Souza and Pedro Paulo de Andrade Santos have viewed and agreed to this submission.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Research Ethics Committee of UFRN, Natal, Brazil (Protocol No. 2.283.894).
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vital, A.G.B., de Carvalho, M.C.M., Maia, C.R. et al. Relationship between tumor thickness and GATA3 immunoexpression in lip and tongue squamous cell carcinomas. Oral Maxillofac Surg (2024). https://doi.org/10.1007/s10006-024-01251-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10006-024-01251-0