Abstract
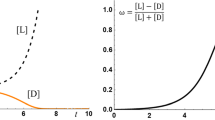
Absolute asymmetric synthesis (AAS) is the preparation of pure (or excess of one) enantiomer of a chiral compound from achiral precursor(s) by a chemical reaction, without enantiopure chiral additive and/or without applied asymmetric physical field. Only one well-characterized example of AAS is known today: the Soai-autocatalysis. In an attempt at clarification of the mechanism of this particular reaction we have undertaken empirical and stochastic analysis of several parallel AAS experiments. Our results show that the initial steps of the reaction might be controlled by simple normal distribution (“coin tossing”) formalism. Advanced stages of the reaction, however, appear to be of a more complicated nature. Symmetric beta distribution formalism could not be brought into correspondence with the experimental observations. A bimodal beta distribution algorithm provided suitable agreement with the experimental data. The parameters of this bimodal beta function were determined by a Pólya-urn experiment (simulated by computer). Interestingly, parameters of the resulting bimodal beta function give a golden section ratio. These results show, that in this highly interesting autocatalysis two or even perhaps three catalytic cycles are cooperating. An attempt at constructing a “designed” Soai-type reaction system has also been made.











Similar content being viewed by others
References
Pasteur L (1848) Mémorie sur la relation qui peut exister entre la forme cristalline et la composition chimique, et sur la cause de la polarisation rotatoire. CR hebdom Séances Acad Sci 26:535–538
Pasteur L (1848) Recherches sur les relations qui peuvent exister entre la forme crystalline, la composition chimique et le sens de la polarisation rotatoire. Ann Chim Phys 24:442–459
Pasteur L (1861) Recherches sur la dissymmetrie des produits organiques naturels. In: Leçons de chimie professées en 1860 par MM Pasteur, Cahours, Wurz, Berthelot, Sainte-Claire Deville, Barral et Dumas. Tome 1, Libraire de L. Hachette, Paris, pp 7 – 48.
Caglioti L, Zucchi C, Florini N, Pályi G (2008) Open Questions about Chirality. In: Pályi G, Zucchi C, Caglioti L (eds) Organometallic chirality. Mucchi – Accad Nazl Sci Lett Arti, Modena, pp 9–27
Flack HD (2009) Louis Pasteur’s discovery of molecular chirality and spontaneous resolution in 1848, together with a complete review of his crystallographic and chemical work. Acta Crystallogr A 65:371–389
Yockey HP (2005) Information theory. Evolution and the origin of life. Cambridge University Press, Cambridge (UK), pp 20–113
Pályi G, Zucchi C, Bencze L, Caglioti L (2004) Biological chirality: a tool of information in vivo and in vitro. In: Seckbach J, Rubin E (eds) New avenues in bioinformatics. Springer, Berlin, pp 81–96
Markó L (2000) Organic chemistry, vol 1. Veszprémi Egyetemi Kiadó, Veszprém, p 48
Pearson K (1898) Chance or Vitalism. Nature 58:495–496
Mills W H (1932) Some aspects of stereochemistry. Chem Ind (London) 750–759.
Frank FC (1953) On spontaneous asymmetric synthesis. Biochim Biophys Acta 11:459–463
Siegel J (1998) Homochiral imperative of life. Chirality 10:24–27
Mason SF (1985) Chemical evolution: origin of biomolecular chirality. Nature 314:400–401
Bonner WA (1988) Origins of chiral homogenity in nature. Top Stereochem 18:1–96
Keszthelyi L (1995) Origin of the homochirality of biomolecules. Q Rev Biophys 28:473–507
Pályi G, Micskei L, Bencze L, Zucchi C (2003) Biological chirality. Magyar Kém Lapja 58:218–223
Fujii N, Saito T (2004) Homochirality and life. Chem Record 4:267–278
Pályi G, Zucchi C, Caglioti L (eds) (2004) Progress in biological chirality. Elsevier, Oxford (UK)
Crick FHC (1968) The origin of the genetic code. J Mol Biol 22:361–363
Yockey H (2002) Information Theory, Evolution and the Origin of Life. In: Pályi G, Zucchi C, Caglioti L (eds) Fundamentals of life. Elsevier, Paris, pp 335–348
Mislow K (2003) Absolute asymmetric synthesis: a commentary. Collect Czechoslov Chem Commun 68:849–864
Pályi G, Micskei K, Zékány L, Zucchi C, Caglioti L (2005) Racemates and the Soai reaction. Magyar Kém Lapja 60:17–24
Crustas J, Hochberg D, Moyano A, Ribó JM (2009) Emergence of chirality in closed systems. ChemPhysChem 10:2123–2131
Sheldon RA (1993) Chirotechnology. Dekker, New York
Soai K, Shibata T, Morioka H, Choji K (1995) Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 378:767–768
Soai K, Shibata T, Sato I (2000) Enantioselective automultiplication of chiral molecules by asymmetric autocatalysis. Acc Chem Res 33:382–390
Soai K, Shibata T, Sato I (2004) Discovery and development of asymmetric autocatalysis. Bull Chem Soc Jpn 77:1063–1073
Soai K, Kawasaki T (2008) Asymmetric autocatalysis with amplification of chirality. Top Curr Chem 284:1–33
Soai K, Kawasaki T (2009) Asymmetric autocatalysis. Automultiplication of chiral molecules. Chem Today 27(6,Suppl): 3–7.
Kawasaki T, Matsumoto A, Soai K (2012) Asymmetric autocatalysis. Pathway to the biological homochirality. Chem Today 30(5):10–13
Soai K, Kawasaki T (2013) Asymmertic autocatalysis of pyrimidyl alkanol. Top Organomet Chem 44:261–280
Soai K, Kawasaki T, Matsumoto A (2014) The origins of homochirality examined using asymmetric catalysis. Chem Record 14:70–83
Pályi G, Zucchi C, Caglioti L (eds) (2012) The Soai reaction and related topic. Artestampa – Accad Nazl Sci Lett Arti, Modena
Soai K, Shibata T, Kowata Y (1997) Production of optically active pyrimidyl alcohol by spontaneous asymmetric synthesis. Jpn Kokai Tokkyo Koho 9,268,179 [Application date: February 1, 1996; April 18, 1996].
Soai K, Sato I, Shibata T, Komiya S, Hayashi M, Matsueda Y, Imamura H, Hayase T, Morioka H, Tabira H, Yamamoto J, Kowata Y (2003) Asymmetric synthesis of pyrimidyl alkanol without adding chiral substances by the addition of diisopropylzinc to pyrimidine-5-carbaldehyde in conjunction with asymmetric autocatalysis. Tetrahedron Asymmetry 14:185–188
Kawasaki T, Suzuki K, Shimizu M, Ishikawa K, Soai K (2006) Spontaneous absolute asymmetric synthesis in the presence of achiral silica gel in conjunction with asymmetric autocatalysis. Chirality 18:479–482
Caglioti L, Hajdu C, Holczknecht O, Zékány L, Zucchi C, Micskei K, Pályi G (2006) The concept of racemates and the Soai reaction. Viva Origino 34:62–80
Caglioti L, Barabás B, Faglioni F, Florini N, Lazzeretti P, Maioli M, Micskei K, Rábai G, Taddei F, Zucchi C, Pályi G (2008) On the track of absolute enantioselective synthesis. Chem Today 26(5):30–32
Barabás B, Caglioti L, Faglioni F, Florini N, Lazzeretti P, Maioli M, Micskei K, Rábai G, Taddei F, Zucchi C, Pályi G (2008) On the traces of absolute enantio selective synthesis. Am Inst Phys Conf Proc 963B:1150–1152
Arnaud GF, Barabás B, Caglioti L, Faglioni F, Florini N, Kocács G, Lazzeretti P, Maioli M, Micskei K, Pályi G, Rábai G, Taddei F, Zucchi C (2012) Towards Absolute Enantioselective Synthesis. In: Zucchi C, Caglioti L (eds) The Soai reaction and related topic (Pályi G. Artestampa – Accad Nazl Sci Lett Arti, Modena, pp 241–273
Shibata T, Morioka H, Hayase T, Choji K, Soai K (1996) A highly enantioselective catalytic asymmetric automultiplication of chiral pyrimidylalcohol. J Am Chem Soc 118:471–472
Shibata T, Yonekubo S, Soai K (1999) Practically perfect asymmetric autocatalysis using 2-alkynyl-5-pyrimidylalkanol. Angew Chem Int Ed 38:659–661
Soai K (2004) Asymmetric autocatalysis, absolute asymmetric synthesis and origin of homochirality of biomolecules. In: Pályi G, Zucchi C, Caglioti L (eds) Progress in biological chirality. Elsevier, Oxford (UK), pp 355–364
Shibata T, Hayase T, Yamamoto J, Soai K (1997) One-pot asymmetric autocatalytic reaction with remarkable amplification of enantiomeric excess. Tetrahedron Asymmetry 8:1717–1719
Sato I, Urabe H, Ishiguro S, Shibata T, Soai K (2003) Amplification of chirality from extremely low to greater than 99.5 % ee by asymmetric autocatalysis. Angew Chem Int Ed 42:315–317
Micskei K, Póta G, Caglioti L, Pályi G (2006) Empirical description of chiral autocatalysis. J Phys Chem A 110:5982–5984
Maioli M, Micskei K, Zucchi C, Caglioti L, Pályi G (2008) Evolution of chirality in consecutive asymmetric autocatalytic reactions. J Math Chem 43:1505–1515
Micskei K, Maioli M, Zucchi C, Caglioti L, Pályi G (2006) Generalization possibilities of autocatalytic absolute enantioselective synthesis. Tetrahedron Asymmetry 17:2960–2962
Buhse T (2003) A tentative kinetic model for chiral amplification in autocatalytic alkylzinc additions. Tetrahedron Asymmetry 14:1055–1061
Micskei K, Rábai G, Gal E, Caglioti L, Pályi G (2008) Oscillatory symmetry breaking in the Soai reaction. J Phys Chem B 112:9196–9200
Caglioti L, Micskei K, Pályi G (2007) Chirality of the very first molecule in absolute enantioselective synthesis. Viva Origino 35:82–84
Barabás B, Caglioti L, Zucchi C, Maioli M, Gál E, Micskei K, Pályi G (2007) Violation of distribution symmetry in statistical evaluation of absolute enantioselective synthesis. J Phys Chem B 111:11506–11510
Rényi A (1970) Probability theory. Akadémiai Kiadó, Budapest
Bartoszynski R, Niewiadomska-Bugaj M (1996) Probability and statistical inference. Wiley, New York
Ross S (2003) Probabilitá e Statistica per l’Ingegneria e le Scienze. Ed. Apogeo, Milano
Asymmetry of weak nuclear forces: Lee T D, Yang C N (1956) Question of parity conservation in weak interactions. Phys Rev 104: 254 – 258. (Errata: (1957) 106: 1371.)
Quack M (2002) How important is parity violation for molecular and biomolecular chirality? Angew Chem Int Ed 41:4618–4630
Quack M, Stohner J, Willeke M (2008) High-resolution spectroscopic studies and theory of parity violation in chiral molecules. Ann Rev Phys Chem 59:741–769
Schwerdtfenger P (2010) The search for parity violation in chiral molecules. In: Computational spectroscopy: methods, experiments and applications (Gruenenberg J, ed.). Wiley-VCH, Weinheim. doi: 10.1002/9783527633272.ch7
Vester F, Ulbricht TLV, Krauch H (1959) Optische Aktivität und Paritätsverletzung im β-Zerfall. Naturwissenschaften 46:68
Yamagata Y (1966) A hypothesis for the asymmetric appearance of biomolecules on earth. J Theor Biol 11:495–498
Mason SF (1984) Origin of biomolecular handedness. Nature 311:19–23
Mason SF, Tranter GE (1984) The parity–violating energy difference between enantiomeric molecules. Mol Phys 53:1091–1111
Tranter GE (1985) The parity violating energy difference between enantiomers of α-amino acids. Mol Phys 56:825–838
Tranter GE (1987) The enantio-preferential stabilization of D–ribose from parity violation. Chem Phys Lett 135:279–282
Kikuchi O, Kiyonaga H (1994) Parity-violating energy shift in helical n-alkanes. J Mol Struct THEOCHEM 312:271–274
Bakasov A, Ha T-K, Quack M (1996) Ab initio calculation of molecular energies including parity-violation interaction. In: Raulin F (ed) Proceedings of the Fourth Trieste Conference on Chemical Evolution: Physics of the Origin and Evolution of Life (Chela-Flores J. Kluwer Academic, Dordrecht, pp 287–296
Zanasi R, Lazzeretti P (1998) On the stabilization of natural L-enantiomers of α-amino acids via parity violating effects. Chem Phys Lett 286:240–242
Zanasi R, Lazzeretti P, Ligabue A, Soncini A (1999) On the stabilization of natural L-α-amino acids and D-sugars via parity-violating effects. In: Zucchi C, Caglioti L (eds) Advances in BioChirality. Elsevier, Amsterdam, pp 377–385
Schwerdtfenger P, Kühn A, Bast R, Laerdahl JK, Faglioni F, Lazzeretti P (2004) The vibrational spectrum of camphor from ab initio functional theory and parity violation in the C-C*-CO bending mode. Chem Phys Lett 383:496–501
Faglioni F, Passalaqua A, Lazzeretti P (2005) Parity violation energy of biomolecules. Orig Life Evol Biosph 35:461–475
Faglioni F, D’Agostino PS, Cadioli B, Lazzeretti P (2005) Parity violation energy of biomolecules. II, DNA. Chem Phys Lett 407:522–526
Faglioni F, Cuesta IG, Lazzeretti P (2006) Parity violation energy of biomolecules. III, RNA. Chem Phys Lett 432:263–268
Faglioni F, Lazzeretti P, Pályi G (2007) Parity violation energy of 5-pyrimidyl alkanol, a chiral autocatalytic molecule. Chem Phys Lett 435:346–349
Faglioni F, Cuesta IG (2011) Parity violation energy of biomolecules. IV, Protein secondary structure. Orig Life Evol Biosph 41:249–259
Lente G (2006) Stochastic analysis of the parity violating energy differences between enantiomers and its implications for the origin of biological chirality. J Phys Chem 110:12711–12713
Lente G (2007) The effect of parity violation on kinetic models of enantioselective autocatalysis. PhysChemChemPhys 9:6134–6141
Lente G (2004) Homogeneous chiral autocatalysis: a simple purely stochastic kinetic model. J Phys Chem A 108:9475–9478
Lente G (2005) Stochastic kinetic models of chiral autocatalysis: a general tool for the quantitative interpretation of total asymmetric synthesis. J Phys Chem A 109:11058–11063
Lente G (2011) Stochastic interpretation of enantiomeric distribution observed in the absolute asymmetric Soai reaction. Tetrahedron Asymmetry 22:1595–1599
Schiaffino L, Ercolani G (2008) Unraveling the mechanism of the Soai asymmetric autocatalytic reaction by first-principles calculations: induction and amplification of chirality by self-assembly of hexanuclear complexes. Angew Chem Int Ed 47:6832–6835
Schiaffino L, Ercolani G (2009) Amplification of chirality and enantioselectivity in the asymmetric autocatalytic Soai reaction. ChemPhysChem 10:2508–2515
Schiaffino L, Ercolani G (2010) Mechanism of the asymmetric autocatalytic Soai reaction studied by density functional theory. Chem Eur J 16:3147–3156
Ercolani G, Schiaffino L (2011) Putting the mechanism of the Soai reaction to the test: DFT study of the role of aldehyde and Dialkylzinc structure. J Org Chem 76:2619–2626
Ercolani G, Schiaffino L (2012) Mechanistic insights into the Soai reaction from formal kinetics and density functional theory calculations. In: Zucchi C, Caglioti L (eds) The Soai Reaction and Related Topic (Pályi G. Artestampa – Accad Nazl Sci Lett Arti, Modena, pp 331–346
Barabás B, Caglioti L, Micskei K, Pályi G (2009) Data-based Stochastic approach to absolute asymmetric synthesis by autocatalysis. Bull Chem Soc Jpn 82:1372–1376
Eggenberger F, Pólya G (1923) Über Statistik verketterter Vorgänge. Z Angew Math Mech 3:279–289
Feller W (1971) An introduction to probability theory and its applications. Wiley, New York, pp 229–230
Varela FJ (1972) Principles of biological autonomy. North Holland, New York, p 172
Hokkyo N (2004) Implication of Polya’s urn experiment in biochirality and cerebral lateralization. In: Pályi G, Zucchi C, Caglioti L (eds) Progress in biological chirality. Elsevier, Oxford, pp 153–158
http://cran.r-project.org/web/packages/polyapost - Description: http://cran.r-project.org/web/packages/polyapost/polyapost.pdf
Reimann J (1989) Mathematical statistics with application in flood hydrology. Akadémiai Kiadó, Budapest. p, 223
Leonardo Fibonacci (Pisa, cca. 1170 – 1250).
Pisano L (2002) Fibonacci’s Liber Abaci (Sigler LE, Transl.). Springer, Berlin
Bóna M (2011) A walk though combinatorics (3rd edn.). World Scientific, Hackensak
Dunlap RA (1997) The golden ratio and Fibonacci numbers. World Scientific, Hackensak
Livio M (2002) The golden ratio: the story of phi, the world’s most astonishing number. Broadway Books, New York
Sato I, Omiya D, Tsukiyama K, Ogi Y, Soai K (2001) Evidence of asymmetric autocatalysis in the enantioselective addition of diisopropylzinc to pyrimidine-5-carbaldehyde using chiral pyrimidyl alcohol. Tetrahedron Asymmetry 12:1965–1969
Blackmond DG, McMillan CR, Ramdeehul S, Schorn A, Brown JM (2001) Origins of asymmetric amplification in autocatalytic alkylzinc additions. J Am Chem Soc 123:10103–10104
Sato I, Omiya D, Igarashi H, Kato K, Ogi K, Tsukiyama K, Soai K (2003) Relationship between the time, yield and enantiomeric excess of asymmetric autocatalysis of chiral 2-alkynyl-5pyrimidyl alkanol with amplification of enantiomeric excess. Tetrahedron Asymmetry 14:975–979
Buono FG, Blackmond DG (2003) Kinetic evidence for a tetrameric transition state in the asymmetric autocatalytic alkylation of pyrimidyl aldehydes. J Am Chem Soc 125:8978–8979
Rivera-Islas J, Lavabre D, Grevy JM, Hernandez-Lamoneta R, Royas-Cabrera H, Micheau J-C, Buhse T (2005) Mirror-symmetry breaking in the Soai reaction: a kinetic understanding. Proc Natl Acad Sci U S A 102:13743–13748
Lavabre D, Micheau J-C, Rivera-Islas J, Buhse T (2008) Kinetic insight into specific features of the autocatalytic Soai reaction. Top Curr Chem 284:67–96
Micheau J-C, Coudret C, Buhse T (2012) Systems chemistry of the Soai reaction. In: Pályi G, Zucchi C, Caglioti L (eds) The Soai reaction and related topic. Artestampa – Accad Nazl Sci Lett Arti, Modena, pp 169–196
Gánti T (1975) Organization of chemical reactions into dividing and metabolizing units. Biosystems 7:15–21
Gánti T (1984) Coupling of autocatalytic cycles as a possible explanation of chemical oscillators. React Kinet Catal Lett 24:197–202
Gánti T (1997) Biogenesis Itself. J Theor Biol 187:583–593
Gánti T (2003a) Chemoton theory, vol. 2, Theory of living systems. Kluwer, Dordrecht
Gánti T (2003) The principle of life. Oxford University Press, Oxford
Eigen M, Schuster P (1977) The hypercycle. A principle of natural self-organization. Part A: emergence of the hypercycle. Naturwissenschaften 64:541–565
Eigen M, Schuster P (1978) The hypercycle. A principle of natural self-organization. Part B: The abstract hypercycle. Naturwissenschaften 65:7–41
Eigen M, Schuster P (1978) The hypercycle. A principle of natural self-organization. Part C: The realistic hypercycle. Naturwissenschaften 65:341–369
Caglioti L, Zucchi C, Pályi G (2005) Single molecule chirality. Chem Today 23(5):38–43
Caglioti L, Pályi G (2008) Chiral chemistry of single molecules. Chem Today 26(3):41–42
Caglioti L, Micskei K, Pályi G (2011) First molecules, biological chirality, origin(s) of life. Chirality 23:65–68
Caglioti L, Pályi G (2013) Single chiral molecule as possible starting element of complex chiral systems. Rend Lincei Sci Fis Nat 24:191–196
Fuss W (2009) Does life originate from a single molecule? Chirality 21:299–304
Fuss W (2009) Biological homochirality as a result from a single event. Colloids Surf B: Biointerface 74:498–503
Carroll JD (2009) A new definition of life. Chirality 21:354–358
Mauksch M, Tsogoeva SB, Martynova IM, Wei S (2007) Evidence for asymmetric autocatalysis in organocatalytic reactions. Angew Chem Int Ed 46:393–396
Mauksch M, Tsogoeva SB, Martynova IM, Wei S (2007) Demonstration of spontaneous chiral symmetry breaking in asymmetric Mannich and aldol reactions. Chirality 19:816–825
Mauksch M, Tsogoeva SB (2008) Spontaneous emergence of homochirality via coherently coupled antagonistic and reversible reaction cycles. ChemPhysChem 9:2359–2372
Mauksch M, Wei S, Freund M, Zamfir A, Tsogoeva SB (2010) Spontaneous mirror symmetry breaking in the aldol reaction and its potential relevance to prebiotic chemistry. Orig Life Evol Biosph 40:79–91
Held FE, Fingerhut A, Tsogoeva SB (2012) Insight into the spontaneous emergence of enantioselectivity in an asymmetric Mannich reaction carried out without external catalyst. Tetrahedron Asymmetry 23:1663–1669
Amedjkouh M, Brandberg M (2008) Asymmetric autocatalytic Mannich reaction in the presence of water and its implication in prebiotic chemistry. Chem Commun 3043–3045
Wang X, Zhang Y, Tan H, Wang Y, Han P, Wang DZ (2010) Enantioselective organocatalytic Mannich reactions with autocatalysis and their mimics. J Org Chem 75:2403–2406
Kovács G, Gyarmati J, Somsák L, Micskei K (1996) Long—lived glycosyl-chromium(III) intermediates in aqueous medium. Preparation of Pyranoid Glycals. Tetrahedron Lett 37:1293–1296
Micskei K, Gyarmati J, Kovács G, Makleit S, Simon C, Szabó Z, Marton J, Hosztafi S, Reinke H, Drexler H-J (1999) Reactions of nepenthone with chromium(II) reagents in neutral aqueous medium. Eur J Org Chem 149–153
Micskei K, Kiss-Szikszai A, Gyarmati J, Hajdu C (2001) Carbon-carbon bond formation in neutral aqueous medium by modification of the Nozaki-Hiyama reaction. Tetrahedron Lett 42:7711–7713
Gyarmati J, Hajdu C, Dinya Z, Micskei K, Zucchi C, Pályi G (1999) Asymmetric induction of amino acid ligands in chromium(II)-assisted reduction of ketones. J Organomet Chem 586:106–109
Patonay T, Hajdu C, Jenkő J, Lévai A, Micskei K, Zucchi C (1999) Enantioselective reduction of prochiral ketones by chromium(II) complexes with amino acid ligands as a source of chirality. Tetrahedron Lett 40:1373–1374
Micskei K, Hajdu C, Wessjohann LA, Mercs L, Kiss-Szikszai A, Patonay T (2004) Enantioselective reduction of prochiral ketones by chromium(II) amino acid complexes. Tetrahedron Asymmetry 15:1735–1744
Micskei K, Holczknecht O, Marchis V, Lévai A, Patonay T, Zucchi C, Pályi G (2005) Enantioselective reduction of C=N double bond by chromium(II) complexes of natural amino acids. Chirality 17:511–514
Micskei K, Holczknecht O, Hajdu C, Patonay T, Marchis V, Meo M, Zucchi C, Pályi G (2003) Asymmetric synthesis of amino acids by Cr(II) complexes of natural amino acids. J Organomet Chem 682:143–148
Micskei K, Patonay T, Caglioti L, Pályi G (2010) Amino acid chirality for enantioselective syntheses. Chem Biodivers 7:1660–1669
Florini N, Arnaud GF, Kónya B, Zucchi C, Pályi G (2009) Synthesis of a water-soluble chiral NMR shift reagent: (S)-PDTA. Tetrahedron Asymmetry 20:1036–1039
Arnaud GF, Florini N, Caglioti L, Zucchi C, Pályi G (2009) Fast enantioselective amino acid quantitative C-13 NMR determination by a praseodymium chiral shift reagent. Tetrahedron Asymmetry 20:1633–1636
Florini N, Faglioni F, Zucchi C, Caglioti L, Pályi G (2010) Aqueous-phase quantitative NMR determination of amino acid enantiomer ratio by C-13 NMR using chiral neodymium shift reagent. Amino Acids 38:1343–1350
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection 6th Conference on Modeling & Design of Molecular Materials in Kudowa Zdrój (MDMM 2014)
Rights and permissions
About this article
Cite this article
Barabás, B., Zucchi, C., Maioli, M. et al. Stochastic and empirical models of the absolute asymmetric synthesis by the Soai-autocatalysis. J Mol Model 21, 33 (2015). https://doi.org/10.1007/s00894-015-2576-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2576-6