Abstract
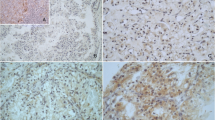
T-cell immunoglobulin and mucin domain (TIMD) family genes are related to innate immune responses. TIMD4 is a receptor for phosphatidylserine and is involved in the phagocytosis of apoptotic cells by macrophages. In the present study, we found that TIMD4 is expressed on the cancer cells of patients with clear cell renal cell carcinoma (ccRCC). TIMD4 was immunostained in the resected samples of 89 patients diagnosed as ccRCC. High expression of TIMD4 in cancer cells was closely related to short progression free survival time; however, it was not correlated with other clinicopathological factors. Intracellular expression of TIMD4 was observed in the RCC cell line, 786-O. In vitro studies using 786-O cells and shRNA targeting TIMD4 indicated that TIMD4 expression was associated with resistance to sorafenib but not with cell proliferation. TIMD4 might be useful as a prognostic factor and may also be a new target for therapy of ccRCC.



Similar content being viewed by others
References
Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH (2010) TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 235:172–189
Kuchroo VK, Dardalhon V, Xiao S, Anderson AC (2008) New roles for TIM family members in immune regulation. Nat Rev Immunol 8:577–580
Cheng L, Ruan Z (2015) Tim-3 and Tim-4 as the potential targets for antitumor therapy. Hum Vaccin Immunother. 11:2458–2462
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S (2007) Identification of Tim4 as a phosphatidylserine receptor. Nature 450:435–439
Baghdadi M, Yoneda A, Yamashina T, Nagao H, Komohara Y, Nagai S, Akiba H, Foretz M, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Takeya M, Viollet B, Yagita H, Jinushi M (2013) TIM-4 glycoprotein-mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance. Immunity 39:1070–1081
Baghdadi M, Jinushi M (2014) The impact of the TIM gene family on tumor immunity and immunosuppression. Cell Mol Immunol 11:41–48
Nakai W, Yoshida T, Diez D, Miyatake Y, Nishibu T, Imawaka N, Naruse K, Sadamura Y, Hanayama R (2016) A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 6:33935
Zhang Q, Wang H, Wu X, Liu B, Liu W, Wang R, Liang X, Ma C, Gao L (2015) TIM-4 promotes the growth of non-small-cell lung cancer in a RGD motif-dependent manner. Br J Cancer 113:1484–1492
Ma C, Komohara Y, Ohnishi K, Shimoji T, Kuwahara N, Sakumura Y, Matsuishi K, Fujiwara Y, Motoshima T, Takahashi W, Yamada S, Kitada S, Nakayama T, Eto M, Takeya M (2016) Infiltration of tumor-associated macrophages is involved in CD44 expression in clear cell renal cell carcinoma. Cancer Sci 107:700–707
Komohara Y, Morita T, Annan DA, Horlad H, Ohnishi K, Yamada S, Nakayama T, Kitada S, Suzu S, Kinoshita I, Dosaka-Akita H, Akashi K, Takeya M, Jinushi M (2015) The coordinated actions of TIM-3 on cancer and myeloid cells in the regulation of tumorigenicity and clinical prognosis in clear cell renal cell carcinomas. Cancer Immunol Res 3:999–1007
Nakagawa T, Ohnishi K, Kosaki Y, Saito Y, Horlad H, Fujiwara Y, Takeya M, Komohara Y (2017) Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin-embedded tissue samples. In press, J Clin Exp Hematop
Mikami S, Oya M, Mizuno R, Kosaka T, Katsube K, Okada Y (2014) Invasion and metastasis of renal cell carcinoma. Med Mol Morphol. 47:63–67
Kuroda N, Tanaka A (2014) Recent classification of renal epithelial tumors. Med Mol Morphol. 47:68–75
Kuroda N, Tanaka A, Ohe C, Nagashima Y (2013) Recent advances of immunohistochemistry for diagnosis of renal tumors. Pathol Int 63:381–390
Li W, Li X, Xu S, Ma X, Zhang Q (2016) Expression of Tim4 in glioma and its regulatory role in LN-18 glioma cells. Med Sci Monit 22:77–82
Kovaleva OV, Samoilova DV, Shitova MS, Gratchev A (2016) Tumor associated macrophages in kidney cancer. Anal Cell Pathol (Amst) 2016:9307549
Komohara Y, Jinushi M, Takeya M (2014) Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 105:1–8
Komohara Y, Takeya M (2017) CAFs and TAMs: maestros of the tumour microenvironment. J Pathol 241:313–315
Heqing Y, Bin L, Xuemei Y, Linfa L (2016) The role and mechanism of autophagy in sorafenib targeted cancer therapy. Crit Rev Oncol Hematol 100:137–140
Kharaziha P, Chioureas D, Baltatzis G, Fonseca P, Rodriguez P, Gogvadze V, Lennartsson L, Björklund AC, Zhivotovsky B, Grandér D, Egevad L, Nilsson S, Panaretakis T (2015) Sorafenib-induced defective autophagy promotes cell death by necroptosis. Oncotarget 6:37066–37082
Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu B, Pan S, Dong X, Tan G, Wei Z, Qiao H, Jiang H, Sun X (2014) Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther 13:1589–1598
Acknowledgments
We thank Ms. Ikuko Miyakawa, Ms. Yui Hayashida, and Mr. Takenobu Nakagawa for their technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI, Nos. 16H05162, 16K11013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no financial competing interests to declare.
Additional information
Hiromu Yano and Takanobu Motoshima contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yano, H., Motoshima, T., Ma, C. et al. The significance of TIMD4 expression in clear cell renal cell carcinoma. Med Mol Morphol 50, 220–226 (2017). https://doi.org/10.1007/s00795-017-0164-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-017-0164-9