Abstract
Objectives
To identify the gene expression of the cytokines IL-9, TNF-α, IL-1, INF-γ, IL-17A, and IL-10 and the chemokines CCL-2/MCP-1 and CCR-6 in the periapical fluid of human root canal infections.
Materials and methods
Twenty samples were collected immediately and 7 days after the cleaning and shaping procedures (after reducing the intracanal microbial load) in an attempt to characterize the expression of these genes. The endogenous expression levels of cytokines and chemokines were analyzed by real-time polymerase chain reaction. The Shapiro-Wilk and the Wilcoxon tests analyzed data.
Results
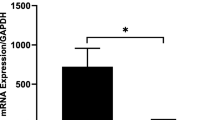
Significantly higher levels of the IL-9, INF-γ, TNF-α, IL-1, and IL-10 markers on day 7 were observed compared with day 0 (p < 0.05). However, IL-17A and the chemokines CCL-2/MCP-1 and CCR-6 did not show a significant difference in mRNA expression when comparing both timepoints (p > 0.05).
Conclusions
The clinical variation of the periapical immune status after endodontic therapy suggests that the cytokine and chemokine-mediated pro-inflammatory response appears to be modulated in an IL-10/IL-9-dependent manner.
Clinical relevance
Few studies have investigated the role of Th9 cells in periapical lesions. IL-9 presents exciting plasticity, performing immunosuppressive actions, and it is also capable of changing their phenotype in the presence of IL-17. Hence, it is relevant to investigate its role in the context of the known mediators involved the periapical immune process.


Similar content being viewed by others
References
Stashenko P, Yu SM, Wang CY (1992) Kinetics of immune cell and bone resorptive responses to endodontic infections. J Endod 18:422–426
Jontell M, Okiji T, Dahlgren U, Bergenholtz G (1998) Immune defense mechanisms of the dental pulp. Crit Rev Oral Biol Med 9:179–200
Sundqvist G (1992) Associations between microbial species in dental root canal infections. Oral Microbiol Immunol 7:257–262
Marton IJ, Kiss C (2014) Overlapping protective and destructive regulatory pathways in apical periodontitis. J Endod 40:155–163
Marton IJ, Kiss C (2000) Protective and destructive immune reactions in apical periodontitis. Oral Microbiol Immunol 15:139–150
Fukada SY, Silva TA, Garlet GP, Rosa AL, da Silva JS, Cunha FQ (2009) Factors involved in the T helper type 1 and type 2 cell commitment and osteoclast regulation in inflammatory apical diseases. Oral Microbiol Immunol 24:25–31
de Brito LCN, Teles FR, Teles RP, Totola AH, Vieira LQ, Ribeiro Sobrinho AP (2012) T-lymphocyte and cytokine expression in human inflammatory periapical lesions. J Endod 38:481–485
Silva TA, Garlet GP, Lara VS, Martins W Jr, Silva JS, Cunha FQ (2005) Differential expression of chemokines and chemokine receptors in inflammatory periapical diseases. Oral Microbiol Immunol 20:310–316
Rossi D, Zlotnik A (2000) The biology of chemokines and their receptors. Annu Rev Immunol 18:217–242
Aranha AM, Repeke CE, Garlet TP et al (2013) Evidence supporting a protective role for Th9 and Th22 cytokines in human and experimental periapical lesions. J Endod 39:83–87
Araujo-Pires AC, Francisconi CF, Biguetti CC, Cavalla F, Aranha AM, Letra A, Trombone AP, Faveri M, Silva RM, Garlet GP (2014) Simultaneous analysis of T helper subsets (Th1, Th2, Th9, Th17, Th22, Tfh, Tr1 and Tregs) markers expression in periapical lesions reveals multiple cytokine clusters accountable for lesions activity and inactivity status. J Appl Oral Sci 22:336–346
Bambirra W Jr, Maciel KF, Thebit MM, de Brito LCN, Vieira LQ, Ribeiro Sobrinho AP (2015) Assessment of apical expression of alpha-2 integrin, heat shock protein, and proinflammatory and immunoregulatory cytokines in response to endodontic infection. J Endod 41:1085–1090
de Brito LCN, Teles FR, Teles RP, Nogueira PM, Vieira LQ, Ribeiro Sobrinho AP (2015) Immunological profile of periapical endodontic infections from HIV- and HIV+ patients. Int Endod J 48:533–541
Henriques LC, de Brito LCN, Tavares WL, Vieira LQ, Ribeiro Sobrinho AP (2011) Cytokine analysis in lesions refractory to endodontic treatment. J Endod 37:1659–1662
Ferreira SB, de Brito LCN, Oliveira MP et al (2015) Periapical cytokine expression in sickle cell disease. J Endod 41:358–362
Kawashima N, Stashenko P (1999) Expression of bone-resorptive and regulatory cytokines in murine periapical inflammation. Arch Oral Biol 44:55–66
Jager A, Kuchroo VK (2010) Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol 72:173–184
Mucida D, Cheroutre H (2010) The many face-lifts of CD4 T helper cells. Adv Immunol 107:139–152
Li H, Rostami A (2010) IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J NeuroImmune Pharmacol 5:198–209
Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ (2009) IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med 206:1653–1660
Tan C, Aziz MK, Lovaas JD et al (2010) Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J Immunol 185:6795–6801
Moller AJ (1966) Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr 74:1–380
Rodrigues RCV, Zandi H, Kristoffersen AK et al (2017) Influence of the apical preparation size and the irrigant type on bacterial reduction in root canal-treated teeth with apical periodontitis. J Endod 43:1058–1063
Barbosa Silva MJ, Vieira LQ, Ribeiro Sobrinho AP (2008) The effects of mineral trioxide aggregates on cytokine production by mouse pulp tissue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:e70–e76
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Cotti E, Schirru E, Acquas E, Usai P (2014) An overview on biologic medications and their possible role in apical periodontitis. J Endod 40:1902–1911
Stessman HA, Bernier R, Eichler EE (2014) A genotype-first approach to defining the subtypes of a complex disease. Cell 156:872–877
Conover E (2015) Environment, more than genetics, shapes immune system. Science 15. https://doi.org/10.1126/science.aaa6349
Takeichi O, Saito I, Tsurumachi T, Moro I, Saito T (1996) Expression of inflammatory cytokine genes in vivo by human alveolar bone-derived polymorphonuclear leukocytes isolated from chronically inflamed sites of bone resorption. Calcif Tissue Int 58:244–248
Bazzoni F, Tamassia N, Rossato M, Cassatella MA (2010) Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: lessons from neutrophils. Eur J Immunol 40:2360–2368
Ivanovski SJ, Farah CS (2007) Alveolar bone loss in T helper 1/T helper 2 cytokine-deficient mice. J Periodontal Res 42:97–103
Colic M, Gazivoda D, Vucevic D et al (2009) Regulatory T-cells in periapical lesions. J Dent Res 88:997–1002
Xing J, Wu Y, Ni B (2011) Th9: a new player in asthma pathogenesis? J Asthma 48:115–125
Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel SL (1996) Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol 157:4602–4608
Lisignoli G, Manferdini C, Codeluppi K, Piacentini A, Grassi F, Cattini L, Filardo G, Facchini A (2009) CCL20/CCR6 chemokine/receptor expression in bone tissue from osteoarthritis and rheumatoid arthritis patients: different response of osteoblasts in the two groups. J Cell Physiol 221:154–160
Santos FR, Moyses RM, Montenegro FL, Jorgetti V, Noronha IL (2003) IL-1beta, TNF-alpha, TGF-beta, and bFGF expression in bone biopsies before and after parathyroidectomy. Kidney Int 63:899–907
Velickovic M, Pejnovic N, Mitrovic S et al (2015) ST2 deletion increases inflammatory bone destruction in experimentally induced periapical lesions in mice. J Endod 41:369–375
Acknowledgments
The authors also wish to thank the post-graduate program at the School of Dentistry of UFMG. LQV and APRS are CNPq fellows.
Funding
The work was supported by the Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (The Research Ethics Committee of the Federal University of Minas Gerais - CAAE: 56942016.2.0000.5149) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maia, L.M., Espaladori, M.C., Diniz, J.M.B. et al. Clinical endodontic procedures modulate periapical cytokine and chemokine gene expressions. Clin Oral Invest 24, 3691–3697 (2020). https://doi.org/10.1007/s00784-020-03247-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03247-y