Abstract
Introduction
Langerhans cell histiocytosis (LCH) is a condition characterized by proliferation of Langerhans cells and wide-range pathologies, ranging from single granulomatous lesions to multi-organ involvement, associated with tissue destruction. LCH pathogenesis remains obscure although association with interleukin (IL)-17A has been reported. We report here a case that illustrates the potential pathogenic role of helper T17 (Th17) cells in LCH-related bone destruction.
Materials and Methods
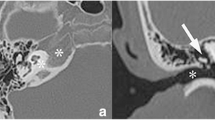
The patient was a 66-year-old woman. The clinical course included craniectomy and bone mass excision in X-9, diagnosis of LCH confirmed by histopathology, followed by 26-month chemotherapy. In August X, the patient was diagnosed with complete central diabetes insipidus. Symptoms improved after treatment with desmopressin. Pituitary magnetic resonance imaging showed swelling extending from the suprasellar region to the pituitary stalk, suggestive of LCH recurrence. This was followed by chemotherapy combined with mercaptopurine hydrate.
Results
Subsequent peripheral blood lymphocyte analysis showed marked increase in activated Th17 cells (CXCR3−CXCR6+ CD4+ T cells). Double staining for CD4 and IL-17 by immunofluorescence of pathological tissue samples obtained during temporal bone mass excision, which confirmed the diagnosis of LCH in X-9, showed areas of combined presence of CD4-positive cells and IL-17-positive cells. Chemotherapy resulted in size reduction of the pituitary lesion and decrease in peripheral blood-activated Th17 cells.
Conclusions
We found abundant peripheral blood-activated Th17 cells and high percentages of IL-17-producing cells in osteolytic bone lesions in LCH. This finding, together with the decrease in peripheral blood-activated Th17 cells following chemotherapy, suggests the potential involvement of activated Th17 cells in LCH-related osteolysis.




Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- LCH:
-
Langerhans cell histiocytosis
- Th17:
-
Helper T17
- IL:
-
Interleukin
- VBL:
-
Vinblastine
- PSL:
-
Prednisolone
- MTX:
-
Methotrexate
- ELISA:
-
Enzyme-linked immunosorbent assay
- OPN:
-
Osteopontin
- TRACP5b:
-
Tartrate-resistant acid phosphatase 5b
- NTX:
-
Cross-linked N-telopeptide of type 1 collagen
References
Liechtenstein L (1953) Histiocytosis X: integration of eosinophilic granuloma, “Letterer-Siwe disease”, and ”Schuller-Christian dis- ease” as related manifestations of a single nosologic entity. AMA Arch Pathol 56:84–102
Girschikofsky M, Arico M, Castillo D, Chu A, Doberauer C et al (2013) Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis 8:72. https://doi.org/10.1186/1750-1172-8-72
Lian C, Lu Y, Shen S (2016) Langerhans cell histiocytosis in adults: a case report and review of the literature. Oncotarget 7:18678–18683. https://doi.org/10.18632/oncotarget.7892
Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F (1997) Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol 28:9–14. https://doi.org/10.1002/(sici)1096-911x(199701)28:1%3c9::aid-mpo3%3e3.0.co;2-p
Grana N (2014) Langerhans cell histiocytosis. Cancer Control 21:328–334. https://doi.org/10.1177/107327481402100409
Cao XX, Duan MH, Zhao AL, Cai H, Chen J et al (2022) Treatment outcomes and prognostic factors of patients with adult Langerhans cell histiocytosis. Am J Hematol 97:203–208. https://doi.org/10.1002/ajh.26412. (Epub 2021 Nov 23)
Malpas JS (1998) Langerhans cell histiocytosis in adults. Hematol Oncol Clin North Am 12:259–268. https://doi.org/10.1016/s0889-8588(05)70509-8
Kubo S, Nakayamada S, Yoshikawa M, Miyazaki Y, Sakata K et al (2017) Peripheral Immunophenotyping identifies three subgroups based on T Cell heterogeneity in lupus patients. Arthritis Rheumatol 69:2029–2037. https://doi.org/10.1002/art.40180
Maecker HT, McCoy JP, Nussenblatt R (2012) Standardizing immunophenotyping for the human immunology project. Nat Rev Immunol 12:191–200. https://doi.org/10.1038/nri3158
Morimoto A, Shioda Y, Imamura T, Kudo K, Kawaguchi H et al (2016) Intensified and prolonged therapy comprising cytarabine, vincristine and prednisolone improves outcome in patients with multisystem Langerhans cell histiocytosis: results of the Japan Langerhans Cell Histiocytosis Study Group-02 Protocol Study. Int J Hematol 104:99–109. https://doi.org/10.1007/s12185-016-1993-3
Egeler RM, Favara BE, van Meurs M, Laman JD, Claassen E (1999) Differential In situ cytokine profiles of Langerhans-like cells and T cells in Langerhans cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood 94:4195–4201
Kobayashi M, Tojo A (2018) Langerhans cell histiocytosis in adults: advances in pathophysiology and treatment. Cancer Sci 109:3707–3713. https://doi.org/10.1111/cas.13817
Ismail MB, Åkefeldt SO, Lourda M, Gavhed D, Aricò M et al (2020) High levels of plasma interleukin-17A are associated with severe neurological sequelae in Langerhans cell histiocytosis. Cytokine 126:154877. https://doi.org/10.1016/j.cyto.2019.154877
Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM (2008) Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol 180:214–221. https://doi.org/10.4049/jimmunol.180.1.214
Aggarwal S, Gurney AL (2002) IL-17: Prototype member of an emerging cytokine family. J Leukoc Biol 71:1–8
Kolls JK, Linden A (2004) Interleukin-17 family members and inflammation. Immunity 21:467–476. https://doi.org/10.1016/j.immuni.2004.08.018
Pöllinger B, Junt T, Metzler B, Walker UA, Tyndall A et al (2011) Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J Immunol 186:2602–2612. https://doi.org/10.4049/jimmunol.1003370
Kamizono J, Okada Y, Shirahata A, Tanaka Y (2002) Bisphosphonate induces remission of refractory osteolysis in Langerhans cell histiocytosis. J Bone Miner Res 17:1926–1928. https://doi.org/10.1359/jbmr.2002.17.11.1926
Oh Y, Morimoto A, Shioda Y, Imamura T, Kudo K, Imashuku S, Japan LCH Study Group (2014) High serum osteopontin levels in pediatric patients with high risk Langerhans cell histiocytosis. Cytokine 70:194–197. https://doi.org/10.1016/j.cyto.2014.07.002
Li N, Cui L, Ma H, Gong Z, Lian H et al (2020) Osteopontin is highly secreted in the cerebrospinal fluid of patient with posterior pituitary involvement in Langerhans cell histiocytosis. Int J Lab Hematol 42:788–795. https://doi.org/10.1111/ijlh.13304
Coury F, Annels N, Rivollier A, Olsson S, Santoro A et al (2008) Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat Med 14:81–87. https://doi.org/10.1038/nm1694
Acknowledgements
We would like to express our sincere gratitude to Dr. Shimajiri Shohei, who performed the pathological staining for this paper.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
KT obtained consent forms. AT and AK collected data. AT wrote the manuscript. YO, AK, KT, SK, and YT reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
A signed consent form was obtained from the patient regarding the publication of the clinical information in an international medical journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tokutsu, A., Okada, Y., Kurozumi, A. et al. Possible involvement of CXCR3-CXCR6 + CD4 + T cells in Langerhans cell histiocytosis. J Bone Miner Metab 41, 212–219 (2023). https://doi.org/10.1007/s00774-022-01397-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01397-5