Abstract
Introduction
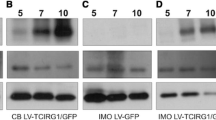
Overexpression studies have been commonly used to yield significant advances in cell biology. In vitro osteoclast culturing involves the differentiation of bone marrow-derived monocyte macrophage precursors (BMMs) in medium supplemented with macrophage colony-stimulating factor and receptor activator of nuclear factor-kB ligand (RANKL) into mature osteoclasts. Retroviral vectors are the gold standards for efficient gene delivery into BMMs. While this strategy is effective in BMMs that are in the early stages of differentiation, it is ineffective in RANKL-treated BMMs such as mono- and multinucleated osteoclasts. This study attempted to enhance gene delivery into differentiated BMMs using liposome-mediated RNA transfection.
Material and methods
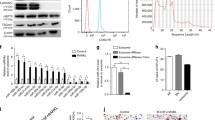
BMMs were transfected with an EYFP overexpression plasmid or EYFP RNA by lipofection, or transduced with a retroviral vector expressing EYFP. EYFP expression was assessed by flow cytometry.
Results
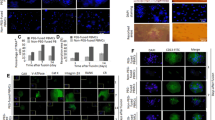
We performed overexpression analyses using enhanced yellow fluorescent protein (EYFP). Although EYFP expression was observed 24 h after infection of BMMs with a recombinant retrovirus containing EYFP, expression of EYFP was observed within 3 h of transfection with EYFP RNA. Moreover, the efficiency of EYFP RNA for gene delivery into BMMs was comparable to that of retroviral transduction of EYFP. In contrast, while very few BMMs stimulated by RANKL for two days expressed EYFP after retroviral infection, more than half of the cells expressed EYFP after transfection with EYFP RNA.
Conclusion
RNA-mediated gene delivery is quick and easy method for performing gain-of-function analyses in primary osteoclast precursors and mature osteoclasts.





Similar content being viewed by others
References
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7:292–304
Lorenzo J, Horowitz M, Choi Y (2008) Osteoimmunology: interactions of the bone and immune system. Endocr Rev 29:403–440
Okamoto K, Takayanagi H (2019) Osteoimmunology. Cold Spring Harbor Perspect Med 9:a03145
Theill LE, Boyle WJ, Penninger JM (2002) RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 20:795–823
Xing L, Schwarz EM, Boyce BF (2005) Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev 208:19–29
Ross FP, Teitelbaum SL (2005) alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev 208:88–105
Jimi E, Shuto T, Koga T (1995) Macrophage colony-stimulating factor and interleukin-1 alpha maintain the survival of osteoclast-like cells. Endocrinology 136:808–811
Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, Shaw AS, Colonna M (2009) Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol 10:734–743
Novack DV, Faccio R (2011) Osteoclast motility: putting the brakes on bone resorption. Ageing Res Rev 10:54–61
Miyazaki T, Sanjay A, Neff L, Tanaka S, Horne WC, Baron R (2004) Src kinase activity is essential for osteoclast function. J Biol Chem 279:17660–17666
Ikeda F, Nishimura R, Matsubara T, Tanaka S, Inoue J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T, Kukita T, Yoshioka K, Rao A, Yoneda T (2004) Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Investig 114:475–484
Gohda J, Akiyama T, Koga T, Takayanagi H, Tanaka S, Inoue J (2005) RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J 24:790–799
Kim JH, Kim K, Jin HM, Song I, Youn BU, Lee SH, Choi Y, Kim N (2010) Negative feedback control of osteoclast formation through ubiquitin-mediated down-regulation of NFATc1. J Biol Chem 285:5224–5231
Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, Fukuda A, Hikita A, Seto H, Okada T, Inaba T, Sanjay A, Baron R, Kawaguchi H, Oda H, Nakamura K, Strasser A, Tanaka S (2003) Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J 22:6653–6664
Tanaka S, Miyazaki T, Fukuda A, Akiyama T, Kadono Y, Wakeyama H, Kono S, Hoshikawa S, Nakamura M, Ohshima Y, Hikita A, Nakamura I, Nakamura K (2006) Molecular mechanism of the life and death of the osteoclast. Ann N Y Acad Sci 1068:180–186
Ikeda F, Matsubara T, Tsurukai T, Hata K, Nishimura R, Yoneda T (2008) JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts. J Bone Mineral Res 23:907–914
Iwasawa M, Miyazaki T, Nagase Y, Akiyama T, Kadono Y, Nakamura M, Oshima Y, Yasui T, Matsumoto T, Nakamura T, Kato S, Hennighausen L, Nakamura K, Tanaka S (2009) The antiapoptotic protein Bcl-xL negatively regulates the bone-resorbing activity of osteoclasts in mice. J Clin Investig 119:3149–3159
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901
Zhao B, Takami M, Yamada A, Wang X, Koga T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, Takayanagi H, Kamijo R (2009) Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med 15:1066–1071
Nishikawa K, Nakashima T, Hayashi M, Fukunaga T, Kato S, Kodama T, Takahashi S, Calame K, Takayanagi H (2010) Blimp1-mediated repression of negative regulators is required for osteoclast differentiation. Proc Natl Acad Sci USA 107:3117–3122
Nishikawa K, Iwamoto Y, Kobayashi Y, Katsuoka F, Kawaguchi SI, Tsujita T, Nakamura T, Kato S, Yamamoto M, Takayanagi H, Ishii M (2015) DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine-producing metabolic pathway. Nat Med 21:281
Nishikawa K, Iwamoto Y, Ishii M (2013) Development of an in vitro culture method for stepwise differentiation of mouse embryonic stem cells and induced pluripotent stem cells into mature osteoclasts. J Bone Miner Metab 32:331
Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, Huang CC, Kain SR (1998) Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem 273:34970–34975
Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ (2006) A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126:995–1004
Lagnado CA, Brown CY, Goodall GJ (1994) AUUUA is not sufficient to promote poly(A) shortening and degradation of an mRNA: the functional sequence within AU-rich elements may be UUAUUUA(U/A)(U/A). Mol Cell Biol 14:7984–7995
Morita S, Kojima T, Kitamura T (2000) Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther 7:1063–1066
Tanaka S, Takahashi T, Takayanagi H, Miyazaki T, Oda H, Nakamura K, Hirai H, Kurokawa T (1998) Modulation of osteoclast function by adenovirus vector-induced epidermal growth factor receptor. J Bone Mineral Res 13:1714–1720
Kobayashi Y, Take I, Yamashita T, Mizoguchi T, Ninomiya T, Hattori T, Kurihara S, Ozawa H, Udagawa N, Takahashi N (2005) Prostaglandin E2 receptors EP2 and EP4 are down-regulated during differentiation of mouse osteoclasts from their precursors. J Biol Chem 280:24035–24042
Matsubara T, Ikeda F, Hata K, Nakanishi M, Okada M, Yasuda H, Nishimura R, Yoneda T (2010) Cbp recruitment of Csk into lipid rafts is critical to c-Src kinase activity and bone resorption in osteoclasts. J Bone Mineral Res 25:1068–1076
Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157:1262–1278
Komor AC, Badran AH, Liu DR (2017) CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell 168:20–36
Mondal P, Krishnamurthy VV, Sharum SR, Haack N, Zhou H, Cheng J, Yang J, Zhang K (2019) Repurposing protein degradation for optogenetic modulation of protein activities. ACS Synth Biol 8:2585–2592
Bonger KM, Rakhit R, Payumo AY, Chen JK, Wandless TJ (2014) General method for regulating protein stability with light. ACS Chem Biol 9:111–115
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS) (18H02614 to K. N.); and grants from the Takeda Science Foundation (to K.N.); and the Food Science Institute Foundation (Ryoushoku-kenkyukai to K. N.); and Suzuken Memorial Foundation (to K. N.); and Terumo Life Science Foundation (to K. N.); and Fuji Foundation For Protein Research (to K. N.); and The Tojuro Iijima Foundation for Food Science and Technology (to K. N.); and Nakatani Foundation (to K. N.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no conflicts of interest.
Ethical approval
Animal study was approved by the Institutional Animal Care and Use Committee of both Doshisha University and Osaka University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nishikawa, K., Ishii, M. Novel method for gain-of-function analyses in primary osteoclasts using a non-viral gene delivery system. J Bone Miner Metab 39, 353–359 (2021). https://doi.org/10.1007/s00774-020-01161-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01161-7