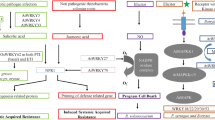
Abstract
To optimize acclimation responses to environmental growth conditions, plants integrate and weigh a diversity of input signals. Signal integration within the signalling networks occurs at different sites including the level of transcription factor activation. Accumulating evidence assigns a major and diversified role in environmental signal integration to the family of APETALA 2/ethylene response element binding protein (AP2/EREBP) transcription factors. Presently, the Plant Transcription Factor Database 3.0 assigns 147 gene loci to this family in Arabidopsis thaliana, 200 in Populus trichocarpa and 163 in Oryza sativa subsp. japonica as compared to 13 to 14 in unicellular algae (http://plntfdb.bio.uni-potsdam.de/v3.0/). AP2/EREBP transcription factors have been implicated in hormone, sugar and redox signalling in context of abiotic stresses such as cold and drought. This review exemplarily addresses present-day knowledge of selected AP2/EREBP with focus on a function in stress signal integration and retrograde signalling and defines AP2/EREBP-linked gene networks from transcriptional profiling-based graphical Gaussian models. The latter approach suggests highly interlinked functions of AP2/EREBPs in retrograde and stress signalling.




Similar content being viewed by others
Abbreviations
- ABA:
-
abscisic acid
- AP2:
-
APETALA 2
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- CBF:
-
C-repeat binding factor
- DREB:
-
dehydration-responsive element binding protein
- EREBP:
-
ethylene response element binding protein
- ERF:
-
ethylene responsive factor
- GGM:
-
graphical Gaussian model
- PSY:
-
phytoene synthase
- RAP2:
-
related to AP2
- RAV:
-
related to ABI3/VP1
- TF:
-
transcription factor
References
Baier M, Ströher E, Dietz KJ (2004) The acceptor availability at photosystem I and ABA control nuclear expression of 2-Cys peroxiredoxin-A in Arabidopsis thaliana. Plant Cell Physiol 45:997–1006
Brazhnik P, de la Fuente A, Mendes P (2002) Gene networks: how to put the function in genomics. Trends Biotechnol 20:467–472
Büttner M, Singh KB (1997) Arabidopsis thaliana ethylene-responsive element binding protein (AtEBP), an ethylene-inducible, GCC box DNA-binding protein interacts with an ocs element binding protein. Proc Natl Acad Sci USA 94:5961–5966
Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leon P, Nambara E, Asami T, Seo M, Koshiba T, Sheen J (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14:2723–2743
Chung S, Parish RW (2008) Combinatorial interactions of multiple cis-elements regulating the induction of the Arabidopsis XERO2 dehydrin gene by abscisic acid and cold. Plant J 54:15–29
Dietz KJ (2008) Redox signal integration: from stimulus to networks and genes. Physiol Plant 133:459–468
Drews GN, Bowman JL, Meyerowitz EM (1991) Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65:991–1002
Feng JX, Liu D, Pan Y, Gong W, Ma LG, Luo JC, Deng XW, Zhu YX (2005) An annotation update via cDNA sequence analysis and comprehensive profiling of developmental, hormonal or environmental responsiveness of the Arabidopsis AP2/EREBP transcription factor gene family. Plant Mol Biol 59:853–868
Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10:1043–1054
Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14:1675–1690
Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M (2000) Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12:393–404
Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 16:433–442
Guo HW, Ecker JR (2004) The ethylene signaling pathway: new insights. Current Opinion Plant Biol 7:40–49
Heber U, Lange OL, Shuvalov VA (2006) Conservation and dissipation of light energy as complementary processes: homoiohydric and poikilohydric autotrophs. J Exp Bot 57:1211–1223
Horling F, Lamkemeyer P, Konig J, Finkemeier I, Kandlbinder A, Baier M, Dietz KJ (2003) Divergent light-, ascorbate-, and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiology 131:317–325
Iwase A, Matsui K, Ohme-Takagi M (2009) Manipulation of plant metabolic pathways by transcription factors. Plant Biotechnol 26:29–38
Jain E, Bairoch A, Duvaud S, Phan I, Redaschi N, Suzek BE, Martin MJ, McGarvey P, Gasteiger E (2009) Infrastructure for the life sciences: design and implementation of the UniProt website. BMC Bioinformatics 10:136
Jofuku KD, Denboer BGW, van Montagu M, Okamuro JK (1994) Control of Arabidopsis flower and seed development by the homeotic gene apetala 2. Plant Cell 6:1211–1225
Kagaya Y, Ohmiya K, Hattori T (1999) RAV1, a novel DNA-binding protein, binds to bipartite recognition sequence through two distinct DNA-binding domains uniquely found in higher plants. Nucleic Acids Res 27:470–478
Karim MR, Hirota A, Kwiatkowska D, Tasaka M, Aida M (2009) A role for Arabidopsis PUCHI in floral meristem identity and bract suppression. Plant Cell 21:1360–1372
Khandelwal A, Elvitigala T, Ghosh B, Quatrano RS (2008) Arabidopsis transcriptome reveals control circuits regulating redox homeostasis and the role of an AP2 transcription factor. Plant Physiol 148:2050–2058
Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J (2007) Multiple signals from damaged chloroplasts converge on a common pathway to regulate nuclear gene expression. Science 316:715–719, Title according to erratum as to 2007-6-22
Krizek BA (2009) AINTEGUMENTA and AINTEGUMENTA-LIKE6 act redundantly to regulate Arabidopsis floral growth and patterning. Plant Physiol 150:1916–1929
Lin RC, Park HJ, Wang HY (2008) Role of Arabidopsis RAP2.4 in regulating light- and ethylene-mediated developmental processes and drought stress tolerance. Mol Plant 1:42–57
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R (2003) ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 15:165–178
Ma S, Bohnert HJ (2008) Gene networks in Arabidopsis thaliana for metabolic and environmental functions. Mol Biosyst 4:199–204
Ma S, Gong Q, Bohnert HJ (2007) An Arabidopsis gene network based on the graphical Gaussian model. Genome Res 17:1614–1625
Magnani E, Sjölander K, Hake S (2004) From endonucleases to transcription factors: evolution of the AP2 DNA binding domain in plants. Plant Cell 16:2265–2277
Manfield IW, Jen CH, Pinney JW, Michalopoulos I, Bradford JR, Gilmartin PM, Westhead DR (2006) Arabidopsis Co-expression Tool (ACT): web server tools for microarray-based gene expression analysis. Nucleic Acids Res 34:W504–W509
Matsui A, Ishida J, Morosawa T, Mochizuki Y, Kaminuma E, Endo TA, Okamoto M, Nambara E, Nakajima M, Kawashima M, Satou M, Kim JM, Kobayashi N, Toyoda T, Shinozaki K, Seki M (2008) Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tilling array. Plant Cell Phys 49(8):1135–1149
Ohme-Takagi M, Shinshi H (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7:173–182
Okamuro JK, Caster B, Villarroel R, Van Montagu M, Jofuku KD (1997) The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc Natl Acad Sci USA 94:7076–7081
Riano-Pachon DM, Ruzicic S, Dreyer I, Mueller-Roeber B (2007) plnTFDB: an integrative plant transcription factor database. BMC Bioinformatics 8:42
Richly E, Dietzmann A, Biehl A, Kurth J, Laloi C, Apel K, Salamini F, Leister D (2003) Covariations in the nuclear chloroplast transcriptome reveal a regulatory master-switch. EMBO reports 4(5):491–498
Rizhsky L, Davletova S, Liang H, Mittler R (2004) The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J Biol Chem 279:11736–11743
Rook F, Bevan MW (2003) Genetic approaches to understanding sugar-response pathways. J Exp Bot 54:495–501
Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW (2001) Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant Journal 26:421–433
Rook F, Hadingham SA, Li Y, Bevan MW (2006) Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ 29:426–434
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290:998–1009
Sauter A, Dietz KJ, Hartung W (2002) A possible stress physiological role of abscisic acid conjugates in root-to-shoot signalling. Plant Cell and Environment 25:223–228
Schäfer J, Strimmer K (2005) A shrinkage approach to large-scale covariance matrix estimation and implications for functional genomics. Stat Appl Genet Mol Biol 4:32
Schwacke R, Fischer K, Ketelsen B, Krupinska K, Krause K (2007) Comparative survey of plastid and mitochondrial targeting properties of transcription factors in Arabidopsis and rice. Mol Genet Genomics 277:631–646
Shaikhali J, Heiber I, Seidel T, Ströher E, Hiltscher H, Birkmann S, Dietz KJ, Baier M (2008) The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol 8:48
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Current Opinion Plant Biol 3:217–223
Song CP, Agarwal M, Ohta M, Guo Y, Halfter U, Wang P, Zhu JK (2005) Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drought stress responses. Plant Cell 17:2384–2396
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94:1035–1040
Ströher E, Dietz KJ (2006) Concepts and approaches towards understanding the cellular redox proteome. Plant Biol 8:407–418
Sun S, Yu JP, Chen F, Zhao TJ, Fang XH, Li YQ, Sui SF (2008) TINY, a dehydration-responsive element (DRE)-binding protein-like transcription factor connecting the DRE- and ethylene-responsive element-mediated signaling pathways in Arabidopsis. J Biol Chem 283:6261–6271
Swarbreck D, Wilks C, Lamesch P, Berardini TZ, Garcia-Hernandez M, Foerster H, Li D, Meyer T, Muller R, Ploetz L, Radenbaugh A, Singh S, Swing V, Tissier C, Zhang P, Huala E (2008) The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucleic Acids Res 36:D1009–D1014
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Toh H, Horimoto K (2002) Inference of a genetic network by a combined approach of cluster analysis and graphical Gaussian modeling. Bioinformatics 18:287–297
The UniProt Consortium (2009) The Universal Protein Resource (UniProt). Nucleic Acids Res 37:D169–D174
Voigt C, Oster U, Börnke F, Jahns P, Dietz KJ, Leister D, Kleine T (2010) In-depth analysis of the distinctive effects of norflurazon implies that tetrapyrrole biosynthesis, organellar gene expression and ABA cooperate in the GUN-type of plastid signalling. Physiol Plant 138:503–519
Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, Frey NFD, Leung J (2008) An update on abscisic acid signaling in plants and more. Molecular Plant 1:198–217
Weigel D (1995) The APETALA2 domain is related to a novel type of DNA binding domain. Plant Cell 7:388–389
Wellmer F, Riechmann JL (2005) Gene network analysis in plant development by genomic technologies. Int J Dev Biol 49:745–759
Welsch R, Medina J, Giuliano G, Beyer P, Von Lintig J (2003) Structural and functional characterization of the phytoene synthase promoter from Arabidopsis thaliana. Planta 216:523–534
Welsch R, Maass D, Voegel T, Dellapenna D, Beyer P (2007) Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiol 145:1073–1085
Wilson K, Long D, Swinburne J, Coupland G (1996) A dissociation insertion causes a semidominant mutation that increases expression of TINY, an Arabidopsis gene related to APETALA2. Plant Cell 8:659–671
Zhuang J, Cai B, Peng RH, Zhu B, Jin XF, Xue Y, Gao F, Fu XY, Tian YS, Zhao W, Qiao YS, Zhang Z, Xiong AS, Yao QH (2008) Genome-wide analysis of the AP2/ERF gene family in Populus trichocarpa. Biochem Biophys Res Com 371:468–474
Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF (2004) Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40:22–34
Zuckerkandl E, Pauling L (1965) Evolutionary divergence and convergence in proteins. In: Bryson V, Vogel HJ (eds) Evolving genes and proteins. Academic Press, New York, pp 97–166
Acknowledgements
The own cited work and analysis was performed within the Special Research Focus FOR804 of the DFG.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on Stress metabolism of plant
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 2
List of genes assigned to graphical Gaussian models (GGM). Table lists names and annotations for genes co-expressed with specified “seed genes”, namely At1g19210, At1g21910, At1g22190, At1g33760, At1g36060, At1g43160, At1g79700, ABI4, At3g14230, At3g50260, At4g34410, At4g39780, At5g18560, At5g47220, At5g51190 and At5g52020. (DOC 455 kb)
Rights and permissions
About this article
Cite this article
Dietz, KJ., Vogel, M.O. & Viehhauser, A. AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma 245, 3–14 (2010). https://doi.org/10.1007/s00709-010-0142-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0142-8