Abstract
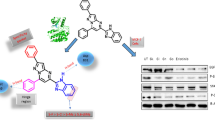
A total of eleven novel 1,2,3-triazole hybrids were synthesized in excellent yields from methyl β-orsellinate through a two-step protocol with 1,3-dipolar cycloaddition as the key step. The newly synthesized compounds have been evaluated for their anti-proliferative potential against a panel of cancer cell lines viz. DU-145, MCF-7, PC-3, IMR-32, and HEK-293T. Interestingly, one of the compounds exhibited higher cytotoxicity on MCF-7 cells with an IC50 of 5 µM as compared to non-cancerous HEK293T cells (23.28 µM). Flow cytometry analysis and acridine orange/ethidium bromide dual staining have showed the significant G0/G1 phase arrest and effective induction of apoptosis, respectively. Furthermore, the inhibition of CDK4/Cyclin D1 complex proteins and thereby downregulation of p-Rb and E2F1 showed that this compound can act as a potent cell-cycle inhibitor. Docking studies also indicated that the compound may act as a strong ATP competitive inhibitor of CDK4/Cyclin D1 complex. Evidently, the methyl β-orsellinate conjugates of 1,2,3-triazole hybrids could be the effective anticancer leads in breast cancer therapeutics.
Graphical abstract







Similar content being viewed by others
References
Harvey AL (2008) Drug Discov 13:894
Rostagno MA, Prado JM (2013) Natural product extraction: principles and applications. Royal Society of Chemistry, p 58
Atanasov AG, Waltenberger B, Pferschy Wenzing EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH (2015) Biotechnol Adv 33:1582
Koehn FE, Carter GT (2005) Nat Rev Drug Discov 4:206
Elix JA, Stocker-Wörgötter E (2008) Nash III TE (Ed.), Lichen biology. Cambridge University Press, Cambridge, p 104
Varol MJ (2015) Appl Pharmacol 8:e105
Dancık V, Seiler KP, Young DW, Schreiber SL, Clemons PA (2010) J Am Chem Soc 132:9259
Huneck S, Yoshimura I (1996) Identification of Lichen substances. Springer, Berlin
Nguyen DMT, Do LMT, Nguyen VT, Chavasiri W, Mortier J, Nguyen PPK (2017) J Nat Prod 80:261
Stocker-Wörgötter E (2008) Nat Prod Rep 25:188
Choudhary MI, Azizuddin, Jalil S (2005) Phytochemistry 66:2346
Lawrey JD (1986) Bryologist 89:111
Boustie J, Grube M (2005) Plant Gen Resour 3:273
Shrestha G, St. Clair LL (2013) Phytochem Rev 12:229
Thadhani VM, Choudhary MI, Ali S, Omar I, Siddique H, Karunaratne V (2011) Nat Prod Res 19:1827
Manojlovic NT, Vasiljevi P, Juskovi M, Najman S, Jankovic S, Andjelkovi (2010) J Med Plants Res 4:817
Huneck S (1999) Naturwissenschaften 86:559
Molnar K, Farkas EZ (2010) Z Naturforsch C 65:157
Buckle DR, Outred DJ, Rockell CJM, Smith H, Spicer BA (1983) J Med Chem 26:251
Giffin MJ, Heaslet H, Brik A, Lin YC, Cauvi G, Wong CH, McRee DE, Elder JH, Stout CD, Torbett BE (2008) J Med Chem 51:6263
Patpi SR, Pulipati L, Yogeeswari P, Sriram D, Jain N, Sridhar B, Murthy R, Anjana DT, Kalivendi SV, Kantevar S (2012) J Med Chem 55:3911
Demaray JA, Thuener JE, Dawson MN, Sucheck SJ (2008) Bioorg Med Chem Lett 18:4868
Singh P, Raj R, Kumar V, Mahajan MP, Bedi PMS, Kaur T, Saxena AK (2012) Eur J Med Chem 47:594
Khan I, Guru SK, Rath SK, Chinthakindi PK, Singh B, Koul S, Bhushan S, Sangwan PL (2016) Eur J Med Chem 108:104
Majeed R, Sangwan PL, Chinthakindi PK, Khan I, Dangroo NA, Thota N, Hamid A, Sharma PR, Saxena AK, Koul S (2013) Eur J Med Chem 63:782
Nguyen C, Kasinathan G, Cortijo IL, Buendia AM, Kaiser M, Brun R, Perez LMR, Johansson NG, Pacanowska DG, Gilbert IH (2005) J Med Chem 48:5942
Yamamoto I, Sekine M, Hata T (1980) J Chem Soc Perkin Trans 1:306
Sasaki T, Minamoto K, Suzuki T, Sugiura T (1979) J Org Chem 44:1424
Ribble D, Goldstein NB, Norris DA, Shellman YG (2005) BMC Biotechnol 5:12
Baker SJ, Reddy EP (2012) Genes Cancer 3:658
Hylands PJ, Ingolfsdottir K (1985) Phytochemistry 24:127
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES (2015) Nat Rev Drug Discov 14:130
Dehong C, Sun X, Zhang X, Cao J (2020) Biomed Res Int 2020:9525207
Dalton S (2015) Trends Cell Biol 25:592
Barvian M (2000) J Med Chem 43:4606
Soni R, Muller L, Furet P, Schoepfer J, Stephan C, Zumstein-Mecker S, Fretz H, Chaudhuri B (2000) Biochem Biophys Res Commun 275:877
Chohan TA, Chen JJ, Qian HY, Pan YL, Chen JZ (2016) Mol BioSyst 12:1250
Roland WC, Smith G, Smith RL (2000) BMJ 320:987
Trott O, Olson AJ (2010) J Comput Chem 31:455
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) J Comput Chem 25:1605
Acknowledgements
The authors acknowledge Y. Suresh for conducting flow cytometry analysis. We acknowledge CSIR-IICT for evaluating the manuscript and providing with communication number IICT/Pubs/2020/331. This work was supported by the Research Fund of DBT, Project Number: SAG-K-120917-0495.
Funding
This article was funded by Council of Scientific and Industrial Research, India, Research Fellowship: STR, VKKM, JJM.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reddy, S.T., Ramakrishna, M., Makani, V.K.K. et al. Synthesis of novel 1,2,3-triazole hybrids of methyl β-orsellinate with capabilities to arrest cell cycle and induce apoptosis in breast cancer cells (MCF-7). Monatsh Chem 153, 461–473 (2022). https://doi.org/10.1007/s00706-022-02922-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-022-02922-y