Abstract
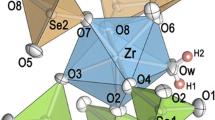
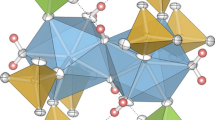
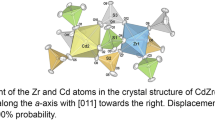
Three new compounds Zr(SeO3)(SeO4), Zr4(SeO3)(SeO4)7, and Zr3(SeO3)(SeO4)5·2H2O were synthesized at low-hydrothermal conditions (Teflon-lined steel vessels, 220 °C) from mixtures of Zr2O2(CO3)(OH)2, H2SeO4, and minor contents of water. Colorless single crystals up to several tenth of a mm in size, obtained within 1 week, were studied by single crystal X-ray techniques. Zr(SeO3)(SeO4) crystallizes in the orthorhombic space group Pbca (No. 61), with a = 8.291 (2) Å, b = 9.458 (2) Å, c = 15.357 (3) Å, V = 1204.2 (5) Å3, Z = 8, R1 = 0.0322. Zr4(SeO3)(SeO4)7 is monoclinic, space group P21/n (No. 14), with a = 5.313 (1) Å, b = 10.704 (2) Å, c = 10.484 (2) Å, β = 104.13 (1)°, V = 578.2 (1) Å3, Z = 1, R1 = 0.0172. Two independent selenium atoms are present in this structure: one forming a SeO4 tetrahedron, and the other one exhibiting mixed occupation by ¾ Se6+ and ¼ Se4+; its coordination is, therefore, partially disordered. Zr3(SeO3)(SeO4)5·2H2O crystallizes in the triclinic space group P1 (No. 1), with a = 5.273 (1) Å, b = 8.079 (2) Å, c = 11.959 (2) Å, α = 82.60 (1)°, β = 88.27 (1)°, γ = 89.87 (1)°, V = 505.1 (1) Å3, Z = 1, R1 = 0.0235, but exhibits strong centrosymmetric pseudosymmetry; the inversion center is violated only by replacement of one selenate(VI) tetrahedron by a trigonal pyramidal selenite(IV) group as pseudo-centric counterpart. Hydrogen bonds in this compound show donor–acceptor distances within the range of 2.67–2.81 Å. All three framework structures are unique and built up from corner-sharing polyhedra. In all three compounds, mean cation-oxygen bond lengths (Zr[6]: 2.062 and 2.067 Å; Zr[7]: 2.132, 2.137 and 2.139 Å; Se4+[3]: 1.675 and 1.680 Å, excluding the disordered group; Se6+[4]: 1.621–1.641 Å) are comparatively short, resulting in rather high bond valence sums.
Graphical abstract





Similar content being viewed by others
References
Effenberger H, Pertlik F (1986) Z Kristallogr 176:75
Giester G, Zemann J (1987) Z Kristallogr 179:431
Giester G (1988) Mineral Petrol 38:277
Giester G (1989) Z Kristallogr 187:239
Giester G, Wildner M (1992) Neues Jahrb Mineral. Monatsh 1992:135
Effenberger H (1996) Acta Chem Scand 50:967
Wildner M, Giester G (2007) Neues Jahrb Mineral. Abh 184:29
Krickl R, Wildner M (2007) Eur J Mineral 19:805
Pristacz H, Talla D, Preuschl F, Giester G, Wildner M (2014) Neues Jahrb Mineral. Abh 191:215
Giester G, Wildner M (2006) Z Kristallogr 221:722
Crichton W, Merlini M, Müller H, Chantel J, Hanfland M (2012) Mineral Mag 76:913
López-Moreno S, Errandonea D, Rodríguez-Hernández P, Muñoz A (2015) Inorg Chem 54:1765
Errandonea D, Muñoz A, Rodríguez-Hernández P, Proctor JE, Sapiña F, Bettinelli M (2015) Inorg Chem 54:7524
Giester G, Wildner M (1991) J Sol State Chem 91:370
Steinhauser G, Luef C, Wildner M, Giester G (2006) J Alloy Compd 419:45
Giester G, Wildner M (2018) Mon Chem 149:1321
Giester G (1989) Mon Chem 120:661
Giester G (1992) Mon Chem 123:957
Giester G (2000) J Alloy Compd 308:71
Brese NE, O’Keeffe M (1991) Acta Crystallogr B 47:192
Zemann J (1986) Z Kristallogr 175:299
Gagné OC, Hawthorne FC (2018) Acta Crystallogr B 74:79
Koskenlinna M (1996) Ann Acad Sci Fenn Chem 262:1
Sheldrick GM (2008) Acta Crystallogr A 64:112
Acknowledgements
This study was financially supported by University Vienna Grants IS526001 and IP532010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wildner, M., Giester, G. Contributions to the stereochemistry of zirconium oxysalts—part II: syntheses and crystal structures of Zr(SeO3)(SeO4), Zr4(SeO3)(SeO4)7, and Zr3(SeO3)(SeO4)5·2H2O. Monatsh Chem 150, 593–603 (2019). https://doi.org/10.1007/s00706-019-2352-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-2352-x