Abstract
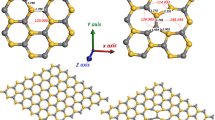
Platinum adsorption on the pristine, Stone–Wales defect, and vacancy defects sites in (8,0) zigzag silicon carbide nanotubes are studied based on the spin-polarized density functional theory. The formation of the Stone–Wales defects with the axial bond rotation is more favorable than the circumferential one. In addition, the vacancy of the carbon atom is more desirable than the silicon atom. The stable adsorption sites and their binding energies on different defect types are analyzed and compared to those on the perfect side wall. It is determined that the adsorption of Pt atom on nine-membered ring in carbon vacancy defect is the most exothermic site. Thus, the presence of intrinsic defects can enhance the reactivity of silicon carbide nanotubes toward Pt atom. Furthermore, the dangling bonds are the main driving force in preventing Pt atom from clustering. It is noticeable that the systems with Pt atom remained semiconductor with direct band gaps. Pt atom on pristine and vacancy-defective silicon carbide nanotubes were positively charged, whereas on Stone–Wales structures, Pt atom gained some charge. In addition, only silicon vacancy structure as structure without Pt atom showed ferromagnetic ordering, while all the systems in presence of Pt atom exhibited non-magnetic moment.
Graphical abstract






Similar content being viewed by others
References
Xi G, He Y, Wang C (2010) Chem Eur J 16:5184
Zhao M, Xia Y, Li F, Zhang R, Lee S-T (2005) Phys Rev B 71:085312
Mpourmpakis G, Froudakis GE, Lithoxoos GP, Samios J (2006) Nano Lett 6:1581
Wu R, Yang M, Lu Y, Feng Y, Huang Z, Wu Q (2008) J Phys Chem C 112:15985
Zhang W, Zhang F, Zhang Z, Lu S, Yang Y (2010) Sci Chin Phys Mech Astron 53:1582
Zhao J-X, Ding Y-H (2008) J Phys Chem C 112:2558
Sun X-H, Li C-P, Wong W-K, Wong N-B, Lee C-S, Lee S-T, Teo B-K (2002) J Am Chem Soc 124:14464
Stone AJ, Wales DJ (1986) Chem Phys Lett 128:501
Pan BC, Yang WS, Yang J (2000) Phys Rev B 62:12652
Orellana W, Fuentealba P (2006) Surf Sci 600:4305
Bettinger HF (2005) J Phys Chem B 109:6922
An W, Wu X, Yang J, Zeng X (2007) J Phys Chem C 111:14105
Jalili S, Akhavan M, Schofield J (2012) J Phys Chem C 116:13225
Jalili S, Molani F, Akhavan M, Schofield J (2014) Physica E 56:48
Wang Z, Gao F, Li J, Zu X, Weber WJ (2009) J Appl Phys 106:084305
Wang X, Liew K (2012) J Phys Chem C 116:26888
Baierle R, Piquini P, Neves LP, Miwa R (2006) Phys Rev B 74:155425
Mao Y-L, Yan X-H, Xiao Y (2005) Nanotechnology 16:3092
Zhang J-M, Wang S-F, Chen L-Y, Xu K-W, Ji V (2010) Eur Phys J B 76:289
Chen YK, Liu LV, Tian WQ, Wang YA (2011) J Phys Chem C 115:9306
Jalili S, Molani F, Schofield J (2013) Can J Chem 91:1
Banerjee S, Nigam S, Pillai C, Majumder C (2012) Int J Hydrogen Energy 37:3733
Wu X, Yang J, Zeng XC (2006) J Chem Phys 125:044704
Tian WQ, Liu LV, Wang YA (2006) Phys Chem Chem Phys 8:3528
Yeung CS, Liu LV, Wang YA (2008) J Phys Chem C 112:7401
Li XM, Tian WQ, Huang X-R, Sun C-C, Jiang L (2009) J Mol Struct Theochem 901:103
Li K, Wang W, Cao D (2011) Sens Actuators B 159:171
Zhang X, Dai Z, Wei L, Liang N, Wu X (2013) Sensor 13:15159
Chen G, Kawazoe Y (2006) Phys Rev B 73:125410
Park Y, Kim G, Lee YH (2008) Appl Phys Lett 92:083108
Gali A (2006) Phys Rev B 73:245415
Dinadayalane TC, Leszczynski J (2007) Chem Phys Lett 434:86
Li Y, Zhou Z, Golberg D, Bando Y, von Ragué Schleyer P, Chen Z (2008) J Phys Chem C 112:1365
Lin T, Wei-De Zhang, Huang J, He C (2005) J Phys Chem B 109:13755
Lu X, Chen Z, Schleyer PvR (2005) J Am Chem Soc 127:20
Chen G-X, Zhang Y, Wang D-D, Zhang J-M (2010) Phys E 43:22
Kittel C (2005) Introduction to solid state physics, 8th edn. Wiley, New York
Wu X, Zeng XC (2006) J Chem Phys 125:44711
Tabtimsai C, Ruangpornvisuti V, Wanno B (2013) Phys E 49:61
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I (2009) J Phys Condens Matter 21:395502
Vanderbilt D (1990) Phys Rev B 41:7892
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Monkhorst HJ, Pack JD (1976) Phys Rev B13:5188
Haddon R (2001) J Phys Chem A 105:4164
Haddon R (1990) J Am Chem Soc 112:3385
Acknowledgments
Computations were performed on the GPC supercomputer at the SciNet HPC Consortium. SciNet is funded by: the Canada Foundation for Innovation under the auspices of Compute Canada; the Government of Ontario; Ontario Research Fund-Research Excellence; and the University of Toronto. Further, we are grateful to Sanandaj Branch, Islamic Azad University Council for the financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molani, F., Jalili, S. & Schofield, J. A computational study of platinum adsorption on defective and non-defective silicon carbide nanotubes. Monatsh Chem 146, 883–890 (2015). https://doi.org/10.1007/s00706-014-1363-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1363-x