Abstract
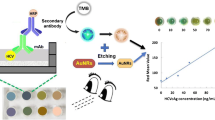
The authors describe a rapid and highly sensitive point-of-care device for rapid determination of noroviruses, a leading cause of acute gastroenteritis. The assay is based on the use of a norovirus-specific aptamer labeled with 6-carboxyfluorescein, and of multi-walled carbon nanotubes (MWCNT) and graphene oxide (GO). The fluorescence of the 6-FAM labeled aptamer is quenched by MWCNT or GO. In the presence of norovirus, fluorescence is recovered due to the release of the labeled aptamer from MWCNT or GO. An easy-to-make paper-based microfluidic platform was developed using a nitrocellulose membrane. The quantitation of norovirus was successfully performed. The linear range extends from 13 ng·mL−1 to 13 μg·mL−1 of norovirus. The detection limits are 4.4 ng·mL−1 and 3.3 ng·mL−1, respectively, when using MWCNT or GO. The device is simple and cost-effective, and holds the potential of rapid in-situ visual determination of noroviruses with remarkable sensitivity and specificity. Hence, it provides a new method for early identification of norovirus and a tool for early intervention when preventing the spread of an outbreak.

ᅟ





Similar content being viewed by others
Abbreviations
- 6-FAM:
-
6-carboxyfluorescein
- ELISA:
-
enzyme-linked immunosorbent assay
- FRET:
-
fluorescence resonance energy transfer
- GO:
-
graphene oxide
- LoD:
-
limit of detection
- LFAs:
-
lateral-flow assays
- MWCNT:
-
multi-walled carbon nanotubes
- POCT:
-
point-of-care testing
- RT-PCR:
-
reverse transcription polymerase chain reaction
References
Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, Koopmans M, Lopman BA (2014) Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infec Dis 14(8):725–730
Koo HL, Ajami N, Atmar RL, DuPont HL (2010) Noroviruses: the principal cause of foodborne disease worldwide. Discov Med 10(50):61
Glass RI, Parashar UD, Estes MK (2009) Norovirus gastroenteritis. N Engl J Med 361(18):1776–1785
Jiang XI, Wang MI, Graham DY, Estes MK (1992) Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol 66(11):6527–6532
Hayashi YU, Ando TA, Utagawa ET, Sekine SE, Okada SH, Yabuuchi K, Miki T, Ohashi M (1989) Western blot (immunoblot) assay of small, round-structured virus associated with an acute gastroenteritis outbreak in Tokyo. J Clin Microbiol 27(8):1728–1733
Vinjé J, Vennema H, Maunula L, von Bonsdorff CH, Hoehne M, Schreier E, Richards A, Green J, Brown D, Beard SS, Monroe SS (2003) International collaborative study to compare reverse transcriptase PCR assays for detection and genotyping of noroviruses. J Clin Microbiol 41(4):1423–1433
Sharma H, Mutharasan R (2013) Review of biosensors for foodborne pathogens and toxins. Sens Actuators B Chem 183:535–549
Hong SA, Kwon J, Kim D, Yang S (2015) A rapid, sensitive and selective electrochemical biosensor with concanavalin A for the preemptive detection of norovirus. Biosens Bioelectron 64:338–344
Hwang HJ, Ryu MY, Park CY, Ahn J, Park HG, Choi C, Ha SD, Park TJ, Park JP (2017) High sensitive and selective electrochemical biosensor: Label-free detection of human norovirus using affinity peptide as molecular binder. Biosens Bioelectron 87:164–170
Ko SM, Kwon J, Vaidya B, Choi JS, Lee HM, Oh MJ, Bae HJ, Cho SY, Oh KS, Kim D (2014) Development of lectin-linked immunomagnetic separation for the detection of hepatitis A virus. Viruses 6(3):1037–1048
Kim J, Adhikari M, Dhamane S, Hagström AE, Kourentzi K, Strych U, Willson RC, Conrad JC (2015) Detection of viruses by counting single fluorescent genetically biotinylated reporter immunophage using a lateral flow assay. ACS Appl Mater Interfaces 7(4):2891
Doerflinger SY, Tabatabai J, Schnitzler P, Farah C, Rameil S, Sander P, Koromyslova A, Hansman GS (2016) Development of a nanobody-based lateral flow immunoassay for detection of human norovirus. mSphere 1(5):e00219–e00216
Wiriyachaiporn N, Sirikett H, Maneeprakorn W, Dharakul T (2017) Carbon nanotag based visual detection of influenza A virus by a lateral flow immunoassay. Microchim Acta 184:1827–1835
Wiriyachaiporn N, Maneeprakorn W, Apiwat C, Dharakul T (2015) Dual-layered and double-targeted nanogold based lateral flow immunoassay for influenza virus. Microchim Acta 182(1–2):85–93
Hagström AE, Garvey G, Paterson AS, Dhamane S, Adhikari M, Estes MK, Strych U, Kourentzi K, Atmar RL, Willson RC (2015) Sensitive detection of norovirus using phage nanoparticle reporters in lateral-flow assay. PLoS One 10(5):e0126571
Davaji B, Lee CH (2014) A paper-based calorimetric microfluidics platform for bio-chemical sensing. Biosens Bioelectron 59:120–126
Zhang Y, Zuo P, Ye BC (2015) A low-cost and simple paper-based microfluidic device for simultaneous multiplex determination of different types of chemical contaminants in food. Biosens Bioelectron 68:14–19
Xu Y, Liu M, Kong N, Liu J (2016) Lab-on-paper micro-and nano-analytical devices: Fabrication, modification, detection and emerging applications. Microchim Acta 183(5):1521–1542
Singh DK, Iyer PK, Giri PK (2012) Role of molecular interactions and structural defects in the efficient fluorescence quenching by carbon nanotubes. Carbon 50(12):4495–4505
Song KM, Lee S, Ban C (2012) Aptamers and their biological applications. Sensors 12(1):612–631
Alig I, Pötschke P, Lellinger D, Skipa T, Pegel S, Kasaliwal GR, Villmow T (2012) Establishment, morphology and properties of carbon nanotube networks in polymer melts. Polymer 53(1):4–28
Huang PJ, Liu J (2012) DNA-length-dependent fluorescence signaling on graphene oxide surface. Small 8(7):977–983
Lin B, Yu Y, Li R, Cao Y, Guo M (2016) Turn-on sensor for quantification and imaging of acetamiprid residues based on quantum dots functionalized with aptamer. Sens Actuators B Chem 229:100–109
Li H, Tian J, Wang L, Zhang Y, Sun X (2011) Multi-walled carbon nanotubes as an effective fluorescent sensing platform for nucleic acid detection. J Mater Chem 21(3):824–828
Chang H, Tang L, Wang Y, Jiang J, Li J (2010) Graphene fluorescence resonance energy transfer aptasensor for the thrombin detection. Anal Chem 82(6):2341–2346
Escudero-Abarca BI, Suh SH, Moore MD, Dwivedi HP, Jaykus LA (2014) Selection, characterization and application of nucleic acid aptamers for the capture and detection of human norovirus strains. PLoS One 9(9):e106805
Mu X, Zhang L, Chang S, Cui W, Zheng Z (2014) Multiplex microfluidic paper-based immunoassay for the diagnosis of hepatitis C virus infection. Anal Chem 86(11):5338–5344
Tian F, Lyu J, Shi J, Yang M (2017) Graphene and graphene-like two-denominational materials based fluorescence resonance energy transfer (FRET) assays for biological applications. Biosens Bioelectron 89:123–135
Thomsen V, Schatzlein D, Mercuro D (2003) Limits of detection in spectroscopy. Spectroscopy 18(12):112–114
Neethirajan S, Ahmed SR, Chand R, Buozis J, Nagy É (2017) Recent advances in biosensor development for foodborne virus detection. Nano 1:272–295
Gupta N, Lainson JC, Belcher PE, Shen L, Mason HS, Johnston SA, Diehnelt CW (2017) Cross-reactive synbody affinity ligands for capturing diverse noroviruses. Anal Chem 89:7174–7181
Todd EC (2016) Foodborne diseases: case Studies of outbreaks in the agri-food industries. CRC Press, (318–240)
Lowther JA, Gustar NE, Hartnell RE, Lees DN (2012) Comparison of norovirus RNA levels in outbreak-related oysters with background environmental levels. J Food Prot 75(2):389–393
Acknowledgements
The authors sincerely thank the Natural Sciences and Engineering Research Council of Canada (400705) for funding this study.
Author information
Authors and Affiliations
Contributions
XW and SN designed the study; XW performed experiments, acquired and analyzed data, XW and SN drafted and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 740 kb)
Rights and permissions
About this article
Cite this article
Weng, X., Neethirajan, S. Aptamer-based fluorometric determination of norovirus using a paper-based microfluidic device. Microchim Acta 184, 4545–4552 (2017). https://doi.org/10.1007/s00604-017-2467-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2467-x