Abstract
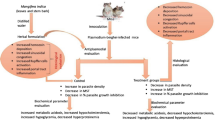
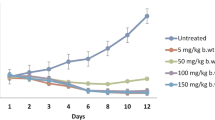
The rate of increasing resistance to most antimalarial drugs suggests a need for better alternatives. Hence, the present study evaluates the in vivo antimalarial and biochemical profiles of a locally formulated herbal antimalarial therapy, Abaleria® on mice infected with Plasmodium berghei. Eight groups of five mice each were used. The control groups include uninfected, infected with 1.0 × 107 P. berghei parasites but not treated, infected, and treated 3 days after inoculation with 25 mg kg−1 chloroquine diphosphate (CDP). Other groups were infected and treated with 50, 100, 200, 300, and 500 mg kg−1/day of Abaleria® for 4 days. On the 5th day, blood smears were prepared and evaluated for parasitemia microscopically, and animals were thereafter sacrificed; serum obtained from blood samples collected through cardiac puncture was used for biochemical assays. There was a significant (p < 0.05) reduction in parasitemia at the highest dose of the drug which compared favorably with CDP. Infection led to elevated liver function indices while treatment with Abaleria® normalized these parameters; a dose dependent increase in HDL-cholesterol was detected in the groups treated with Abaleria® and CDP. The study shows that Abaleria® displayed a dose-dependent in vivo antiplasmodial and biochemical properties as well as improvement of lipid profiles of mice infected with P. berghei.


Similar content being viewed by others
References
Abdulelah HA, Hesham MA, Adel AA, Rohela M (2011) Antimalarial activity of methanolic leaf extract of Piperbetle leaves. Molecules 16:107–118
Adebayo JO, Krettli AU (2011) Potential anti-malarials from Nigerian plants: a review. J Ethnopharmacol 133:289–302
Adebayo AH, Yakubu OF, Adegbite OS, Okubena O (2017) Haematopoietic induction and hepatic protective roles of Hepacare® in CCl4 induced hepatic damaged rats. Comp Clin Pathol 26:679–688
Adekunle AS, Adekunle OC, Egbewale BE (2007) Serum status of selected biochemical parameters in malaria: an animal model. Biol Res 18:109–113
Ajaiyeoba E, Falade M, Ogbole O, Okpako L, Akinboye D (2006) In vivo antimalarial activity and cytotoxic properties of Annona senegalensis extract. Afr J Tradit Complement Altern Med 3:137–141
Akanbi OM (2013) In vivo study of anti-plasmodia activity of Terminalia avicennioides and its effect on Lipid profile and oxidative stress in mice infected with Plasmodium berghei. Br Microbiol Res J 3(4):501–512
Akanbi OM, Omonkhua AA, Cyril-Olutayo CA, Fasimoye RY (2012) The anti-plasmodial activity of Anogeissus leiocarpus and its effect on oxidative stress and lipid profile in mice infected with Plasmodium berghei. Parasitol Res 110:219–226
Alencar NM, Figueiredo IS, Vale MR, Bitencurt FS, Oliveira JS, Ribeiro RA, Ramos MV (2004) Anti-inflammatory effect of the latex from Calotropis procera in three different experimental models: peritonitis, paw edema and hemorrhagic cystitis. Planta Med 70:1144–1149
Barik T (2015) Antimalarial drug: from its development to deface. Curr Drug Discov Technol 12(4):225–228
Beckwith R, Schenkel RH, Silverman PH (1975) Qualitative analysis of phospholipids isolated from nonviable Plasmodium antigen. Exp Parasitol 37(2):164–172
Bergmeyer HU, Herder M, Rej R (1986a) International Federation of Clinical Chemistry (IFCC) scientific committee, analytical section: approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase (L-aspartate: 2-oxoglutarate aminotransferase, EC 2.6 1:1). J Clin Chem Clin Biochem 24(7):497–510
Bergmeyer HU, Herder M, Rej R (1986b) International Federation of Clinical Chemistry (IFCC) scientific committee, analytical section: approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 3. IFCC method for alanine aminotransferase (L-alanine: 2-oxoglutarate aminotransferase, EC 2.6 1:2). J Clin Chem Clin Biochem 24(7):481–495
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31(1):87–96
Doumas BT, Perry BW, Sasse EA, Straumfjord JV Jr (1973) Standardization in bilirubin assays: evaluation of selected methods and stability of bilirubin solutions. Clin Chem 19(9):984–993
Fabbri C, de Cassia Mascarenhas-Netto R, Lalwani P, Melo GC, Magalhaes BML et al (2013) Lipid peroxidation and antioxidant enzymes activity in Plasmodium vivax malaria patients evolving with cholestatic jaundice. Malar J 12:315. https://doi.org/10.1186/1475-2875-12-315
Gonçalves LA, Cravo P, Ferreira MU (2014) Emerging Plasmodium vivax resistance to chloroquine in South America: an overview. Mem Inst Oswaldo Cruz 109(5):534–539
Iweala EEJ, Odidoa O (2009) Effect of a long term consumption of a diet supplemented with leaves of Gongronema latifolium (Benth.) on some biochemical and histological parameters in male albino rats. J Biol Sci 9:859–865
Kanerkar UR, Bhogaonkar PY, Indurwade NH (2015) Antispermatogenic effect of Caesalpinia bonduc (L.) Roxb. Seeds. Int Res J Sci Eng 3(4):173–178
Kannur DM, Paranjpe MP, Sonavane LV, Dongree PP, Khandelwal KR (2012) Evaluation of Caesalpinia bonduc seed coat extract for anti-inflammatory and analgesic activity. J Adv Pharm Technol Res 3(3):171–175
Krishna AP, Chandrika SA, Manasa A, Shrikant LP (2009) Variation in common lipid parameters in malaria infected patients. Ind J Pharmacol 53:271–274
Maroyi A (2013) Traditional use of medicinal plants in south-central Zimbabwe: review and perspectives. J Ethnobiol Ethnomed 9:31–49
Moghadamtousi SZ, Fadaeinasab M, Nikzad S, Mohan G, Ali HM, Kadir HA (2015) Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci 16(7):15625–15658
Mohanty S, Mishra SK, Das BS, Satpathy SK, Mohanty D, Patnaik JK, Bose TK (1992) Altered plasma lipid pattern in falciparum malaria. Ann Trop Med Parasitol 86:601–606
Nawal MA, Ramzi AM, Matheeussen A, Paul PC (2012) In vitro antiplasmodial, antileishmanial and antitrypanosomal activities of selected medicinal plants used in the traditional Arabian Peninsula region. BMC Complement Altern Med 12:49
Newman DJ, Cragg GM, Snader KM (2003) Natural products as sources of new drugs over the period of 1981-2002. J Nat Prod 66:1022–1037
Nicholas JW (2004) Antimalarial drug resistance. J Clin Invest 113:1084–1092
Ogbonna DN, Sokari TG, Agomuoh AA (2008) Antimalarial activities of some selected traditional herbs from South Eastern Nigeria against Plasmodium species. Res J Parasitol 3:25–31
Ogunlana OO, Kim H, Wataya Y, Olagunju JO, Akindahunsi AA, Tan NH (2015) Antiplasmodial flavonoid from young twigs and leaves of Caesalpinia bonduc (Linn) Roxb. J Chem Pharm Res 7(1):931–937
Ojurongbe O, Ojo JA, Adefokun DI, Abiodun OO, Odewale G, Awe EO (2015) In vivo antimalarial activities of Russelia equisetiformis in Plasmodium berghei infected mice. Ind J Pharm Sci 77(4):504–510
Ovenden JR, Morgan JAT, Street R, Tobin A, Simpfendorfer C, Macbeth W, Welch D (2011) Negligible evidence for regional genetic population structure for two shark species Rhizoprionodon acutus and Sphyrna lewini with contrasting biology. Mar Biol 158:1497–1509
Petros Z (2011) The need of standardized herbal remedies as alternate sources of antimalarial products in Ethiopia - updated review. Pharmacology 3:1440–1447
Philipson JD, Wright CW (1990) Anti-protozoal compound from plant source. Planta Med 57:553–559
Ramazani A, Zakeri S, Sardari S, Astaran KN, Djadidt N (2010) In vitro and in vivo anti-malarial activity of Boerhavia elegans and Solanum surattense. Malar J 9:124
Somsak V, Noilod J, Chachiyo S, Kraithep S (2015) Antimalarial activity of ethanolic leaf extract of Bauhinia strychnifolia in mice infected with Plasmodium berghei. Malar Cont Elimination 4:131. https://doi.org/10.4172/2470-6965.1000131
Somsak V, Borkaew P, Klubsri C, Dondee K, Bootprom P, Saiphet B (2016a) Antimalarial properties of aqueous crude extracts of Gynostemma pentaphyllum and Moringa oleifera leaves in combination with artesunate in Plasmodium berghei infected mice. J Trop Med 2016:1–6. https://doi.org/10.1155/2016/8031392
Somsak V, Polwiang N, Chachiyo S (2016b) In vivo antimalarial activity of Annona muricata leaf extract in mice infected with Plasmodium berghei. Journal of Pathogens 2016:1–5. https://doi.org/10.1155/2016/3264070
Tietz NW, Rinker AD, Shaw LM (1983) International Federation of Clinical Chemistry. IFCC methods for the measurement of catalytic concentration of enzymes, Part 5. IFCC method for alkaline phosphatase (orthophosphoric-monoester phosphohydrolase, alkaline optimum, EC 3.1.3.1). J Clin Chem Clin Biochem 21(11):731–748
Viriyavejakul P, Vasant K, Chuchard P (2014) Liver changes in severe Plasmodium malaria: histopathology, apopotosis, and nuclear factor kappa B expression. Malar J 13:106. https://doi.org/10.1186/1475-2875-13-106
White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM (2014) Malaria. Lancet 383(9918):723–735
Wichmann O, Muhlen M, Grub H, Mockenhaupt FP, Suttorp N, Jelinek T (2004) Malarone treatment failure not associated with previously described mutations in the cytochrome-b gene. Malar J 3:14
World Health Organization (2012) World malaria report. Geneva, Switzerland
World Health Organization (2014) Malaria in Africa. Roll Back Malaria Infosheet
World Health Organization (2016) Monitoring health for sustainable development goals. World Health Statistics, France, Europe
Zofou D, Nyasa RB, Nsagha DS, Ntie-Kang F, Meriki HD, Assob JCN, Kuete V (2014) Control of malaria and other vector borne protozoan diseases in the tropics: enduring challenges despite considerable progress and achievements. Infect Dis Poverty 3(1):1
Zoppi F, Fellini D (1976) Enzymatic colorimetric cholesterol determination. Clin Chem 22:690–691
Acknowledgements
The authors appreciate the support of the technical staff of the Department of Biochemistry, Covenant University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The research was approved by the Ethics Committee of the Department of Biological Sciences Covenant University, Nigeria. All animals were also treated in line with the National institute of Health (NIH) guidelines for the use and care for animals in the laboratory (NIH 2011).
Rights and permissions
About this article
Cite this article
Adebayo, A.H., Yakubu, O.F., Popoola, J.O. et al. Evaluation of antimalarial and biochemical profiles of Abaleria® in Plasmodium berghei-infected mice. Comp Clin Pathol 27, 1595–1601 (2018). https://doi.org/10.1007/s00580-018-2780-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2780-8