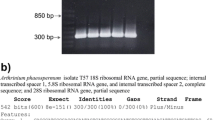
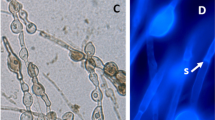
Abstract
The ectomycorrhizal basidiomycete Tricholoma matsutake associates with members of the Pinaceae such as Pinus densiflora (red pine), forming a rhizospheric colony or “shiro,” which produces the prized “matsutake” mushroom. We investigated whether the host specificity of T. matsutake to conifers is innately determined using somatic plants of Cedrela odorata, a tropical broad-leaved tree (Meliaceae) that naturally harbors arbuscular mycorrhizal fungi. We found that T. matsutake could form in vitro shiro with C. odorata 140 days after inoculation, as with P. densiflora. The shiro was typically aromatic like that of P. densiflora. However, this was a root endophytic interaction unlike the mycorrhizal association with P. densiflora. Infected plants had epidermal tissues and thick exodermal tissues outside the inner cortex. The mycelial sheath surrounded the outside of the epidermis, and the hyphae penetrated into intra- and intercellular spaces, often forming hyphal bundles or a pseudoparenchymatous organization. However, the hyphae grew only in the direction of vascular bundles and did not form Hartig nets. Tricholoma fulvocastaneum or “false matsutake” naturally associates with Fagaceae and was also able to associate with C. odorata as a root endophyte. With T. matsutake, C. odorata generated a number of roots and showed greatly enhanced vigor, while with T. fulvocastaneum, it generated a smaller number of roots and showed somewhat lesser vigor. We argue that the host–plant specificity of ectomycorrhizal matsutake is not innately determined, and that somatic arbuscular mycorrhizal plants have a great potential to form mutualistic relationships with ectomycorrhizal fungi.






Similar content being viewed by others
References
Ashford AE, Allaway WG (1982) A sheathing mycorrhiza on Pisonia grandis R. Br. (Nyctaginaceae) with development of transfer cells rather than a Hartig net. New Phytol 90:511–519
Beauchamp VB, Stromberg JC, Stutz JC (2006) Arbuscular mycorrhizal fungi associated with Populus-Salix stands in a semiarid riparian ecosystem. New Phytol 170:369–380
Brundertt MC, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with mycorrhizas in forestry and agriculture. Australian Centre for International Agricultural Research, Canberra, Australia, pp 1–374
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304
Bruns TD, Bidartondo MI, Taylor DL (2002) Host specificity in ectomycorrhizal communities: what do the exceptions tell us. Integr Comp Biol 42:352–359
Danell E, Camacho FJ (1997) Successful cultivation of the golden chanterelle. Nature 385:303
Godbout C, Fortin FA (1985) Synthesized ectomycorrhizae of aspen: fungal genus level of structural characterization. Can J Bot 63:252–262
Guerin-Laguette A, Matsushita N, Lapeyrie F, Shindo K, Suzuki K (2005) Successful inoculation of mature pine with Tricholoma matsutake. Mycorrhiza 15:301–305
Hall IR, Wang Y, Amicucci A (2003) Cultivation of edible ectomycorrhizal mushrooms. Trends Biotechnol 21:433–438
Harley JL, Smith SE (1983) Mycorrhizal symbiosis. Academic, London, UK, pp 1–483
Haug I, Lempe J, Homeier J, Weiß M, Setaro S, Oberwinkler F, Kottke I (2004) Graffenrieda emarginata (Melastomataceae) forms mycorrhizas with Glomeromycota and with a member of the Hymenoscyphus ericae aggregate in the organic soil of a neotropical mountain rain forest. Can J Bot 82:340–356
Haug I, Weiß M, Homeier J, Oberwinkler F, Kottke I (2005) Russulaceae and Thelephoraceae form ectomycorrhizas with members of the Nyctaginaceae (Caryophyllales) in the tropical mountain rain forest of southern Ecuador. New Phytol 165:923–936
Hosford D, Pilz D, Molina R, Amaranthus M (1997) Ecology and management of the commercially harvested American matsutake mushroom. USDA Forest service PNW-GTR-412
Langer I, Krpata D, Peintner U, Winzel WW, Schweiger P (2008) Media formulation influences in vitro ectomycorrhizal synthesis on the European aspen Populus tremula L. Mycorrhiza 18:297–307
Lian C, Narimatsu M, Nara K, Hogetsu T (2006) Tricholoma matsutake in a natural Pinus densiflora forest: correspondence between above- and below-ground genets, association with multiple host trees and alteration of existing ectomycorrhizal communities. New Phytol 171:825–836
Maruyama TE (2009) Polyethylene glycol improves somatic embryo maturation in big-leaf mahogany (Swietenia macrophylla King, Meliaceae). Bull FFPRI 8:167–173
Maruyama E, Ishii K, Sato A, Migita K (1989) Micropropagation of cedro (Cedrela odorata L.) by shoot-tip culture. J Jpn For Soc 71:329–331
Maruyama E, Hosoi Y, Ishii K (2005) Propagation of Japanese red pine (Pinus densiflora Zieb. et Zucc.) via somatic embryogenesis. Propag Ornam Plants 5:199–204
Molina R, Massicotte H, Trappe JM (1992) Specificity phenomena in mycorrhizal symbiosis: community-ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning: an integrative plant-fungal process. Chapman and Hall, New York, pp 357–423
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murata H, Yamada A, Babasaki K (1999) Identification of repetitive sequences containing motifs of retrotransposons in the ectomycorrhizal basidiomycete Tricholoma matsutake. Mycologia 91:766–775
Murata H, Ohta A, Yamada A, Narimatsu M, Futamura N (2005) Genetic mosaics in the massive persisting rhizosphere colony “shiro” of the ectomycorrhizal basidiomycete Tricholoma matsutake. Mycorrhiza 15:505–512
Ogawa M (1976) Microbial ecology of ‘Shiro’ in Tricholoma matsutake (S. Ito et Imai) Sing. and its allied species. III Tricholoma matsutake in Picea glehnii and Picea glehnii-Abies sachalinensis forests. Trans Mycol Soc Japan 17:188–198
Ogawa M (1977) Microbial ecology of ‘Shiro’ in Tricholoma matsutake (S. Ito et Imai) Sing. and its allied species. IV Tricholoma matsutake in Tsuga diversifolia forests. Trans Mycol Soc Jpn 18:20–33
Ogawa M (1978) The biology of matsutake. Tsukiji-shokan, Tokyo (Japanese), pp 1–333
Ohta A (1994) Production of fruit-bodies of a mycorrhizal fungus, Lyophyllum shimeji, in pure culture. Mycoscience 35:147–151
Ohta A (1998) Fruit-body production of two ectomycorrhizal fungi in the genus Hebeloma in pure culture. Mycoscience 39:15–19
Ohta A, Fujiwara N (2003) Fruit-body production of an ectomycorrhizal fungus in genus Boletus in pure culture. Mycoscience 44:295–300
Ota Y, Yamanaka T, Murata H, Neda H, Ohta A, Kawai M, Yamada A, Konno M, Tanaka C (2012) Phylogenetic relationship and species delimitation of “matsutake” and allied species based on multilocus phylogeny and haplotype analyses. Mycologia. doi:10.3852/12-068
Peterson RL, Massicotte HB, Melville LH (2004) Mycorrhizas: anatomy and cell biology. CABI Publishing, Ottawa, pp 1–173
Rooney DC, Prosser JI, Bending GD, Baggs EM, Killham K, Hodge A (2011) Effect of arbuscular mycorrhizal colonisation on the growth and phosphorus nutrition of Populus euramericana c.v. Ghoy. Biomass Bioenergy 35:4605–4612
Sanmee R, Lumyong P, Dell B, Lumyong S (2010) In vitro cultivation and fruit body formation of the black bolete, Phlebopus portentosus, a popular edible ectomycorrhizal fungus in Thailand. Mycoscience 51:15–22
Shi ZY, Chen YL, Feng G, Liu RJ, Christie P, Li XL (2006) Arbuscular mycorrhizal fungi associated with the Meliaceae on Hainan island, China. Mycorrhiza 16:81–87
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic/Elsevier, London, UK, pp 1–787
Urgiles N, Loján P, Aguirre N, Blaschke H, Günter S, Stimm B, Kottke I (2009) Application of mycorrhizal roots improves growth of tropical tree seedlings in the nursery: a step towards reforestation with native species in the Andes of Ecuador. New Forest 38:229–239
Vaario LM, Pennanen T, Sarjala T, Savonen EM, Heinonsalo J (2010) Ectomycorrhization of Tricholoma matsutake and two major conifers in Finland–an assessment of in vitro mycorrhiza formation. Mycorrhiza 20:511–518
Vozzo JA, Hacskaylo E (1974) Endo- and ectomycorrhizal associations in five Populus species. Bull Torrey Bot Club 101:182–186
Yamada A, Maeda K, Ohmada M (1999) Ectomycorrhizal formation of Tricholoma matsutake isolates on seedlings of Pinus densiflora in vitro. Mycoscience 40:455–463
Yamada A, Maeda K, Kobayashi H, Murata H (2006) Ectomycorrhizal symbiosis in vitro between Tricholoma matsutake and Pinus densiflora seedlings that resembles naturally occurring ‘shiro’. Mycorrhiza 16:111–116
Yamada A, Kobayashi H, Murata H, Kalmiş E, Kalyoncu F, Fukuda M (2010) In vitro ectomycorrhizal specificity between the Asian red pine Pinus densiflora and Tricholoma matsutake and allied species from worldwide Pinaceae and Fagaceae forests. Mycorrhiza 20:333–339
Acknowledgements
This work was supported by grants from the Institute for Fermentation, Osaka, Japan, and from the Forestry and Forest Products Research Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murata, H., Yamada, A., Maruyama, T. et al. Root endophyte interaction between ectomycorrhizal basidiomycete Tricholoma matsutake and arbuscular mycorrhizal tree Cedrela odorata, allowing in vitro synthesis of rhizospheric “shiro”. Mycorrhiza 23, 235–242 (2013). https://doi.org/10.1007/s00572-012-0466-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-012-0466-7