Abstract
Background
Localized gastric cancer (GC) becomes fatal once recurring. We still have room for improving their prognoses.
Methods
Transcriptomic analysis was done on surgically resected specimens of 16 patients with UICC stage III GC who underwent curative gastrectomy and adjuvant oral fluoropyrimidine monotherapy. Four of them were free from disease for longer than 5 years, and the others experienced metachronous metastasis within 2 years after surgery. Quantitative RT-PCR determined mRNA expression levels of primary gastric cancer tissues, which were collected from 180 patients who underwent gastric resection for stage II–III GC without preoperative treatment between 2001 and 2014. We tested alteration of malignant phenotypes including drug resistance of GC cell lines by siRNA and shRNA-mediated knockdown and forced expression experiments.
Results
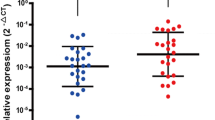
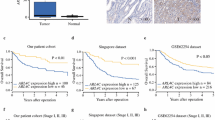
CPLX1 was identified as a candidate biomarker for GC recurrence among 57,749 genes. Inhibiting and forced expression experiments indicated that CPLX1 promotes proliferation, motility, and invasiveness of GC cells, and decreases apoptosis and sensitivity to fluorouracil. Subcutaneous xenograft mouse models revealed that shRNA-mediated knockdown of CPLX1 also attenuated tumor growth of MKN1 cells in vivo. Overexpression of CPLX1 in gastric cancer tissue correlated with worse prognosis and was an independent risk factor for peritoneal recurrence in subgroups receiving adjuvant chemotherapy.
Conclusions
CPLX1 may represent a biomarker for recurrence of gastric cancer and a target for therapy.




Similar content being viewed by others
Abbreviations
- EMT:
-
Epithelial–mesenchymal transition
- mRNA:
-
Messenger RNA
- OD:
-
Optical density
- qRT-PCR:
-
Quantitative reverse-transcription polymerase chain reaction
- RNA-seq:
-
RNA-sequencing
- siRNA:
-
Small interfering RNA
- shRNA:
-
Small hairpin RNA
- VC:
-
Vesicular cycle
References
Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75.
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53.
Hiki N, Katai H, Mizusawa J, et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG0703). Gastric Cancer. 2018;21:155–61.
Shimizu D, Kanda M, Kodera Y, et al. Cutting-edge evidence of adjuvant treatments for gastric cancer. Expert Rev Gastroenterol Hepatol. 2018;12:1109–22.
Knight G, Earle CC, Cosby R, et al. Neoadjuvant or adjuvant therapy for resectable gastric cancer: a systematic review and practice guideline for North America. Gastric Cancer. 2013;16(1):28–40.
Pelcovits A, Almhanna K. Locoregional gastric cancer: a narrative review of multidisciplinary management. Ann Transl Med. 2020;8:1108.
Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95.
Tolios A, De Las RJ, Hovig E, et al. Computational approaches in cancer multidrug resistance research: Identification of potential biomarkers, drug targets and drug-target interactions. Drug Resist Updat. 2020;48: 100662.
Tan P, Yeoh KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149(1153–62): e3.
Brosnan JA, Iacobuzio-Donahue CA. A new branch on the tree: next-generation sequencing in the study of cancer evolution. Semin Cell Dev Biol. 2012;23:237–42.
Redler S, Strom TM, Wieland T, et al. Variants in CPLX1 in two families with autosomal-recessive severe infantile myoclonic epilepsy and ID. Eur J Hum Genet. 2017;25:889–93.
Nagy Á, Lánczky A, Menyhárt O, et al. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8:9227.
Tanaka H, Kanda M, Miwa T, et al. Pattern-specific transcriptomics identifies ASGR2 as a predictor of hematogenous recurrence of gastric cancer. Mol Cancer Res. 2018;16:1420–9.
Tang M, Sun J, Shimizu K, et al. Evaluation of methods for differential expression analysis on multi-group RNA-seq count data. BMC Bioinform. 2015;16:361.
Tanaka H, Kanda M, Shimizu D, et al. FAM46C serves as a predictor of hepatic recurrence in patients with resectable gastric cancer. Ann Surg Oncol. 2016;24:3438–45.
Tanaka HKM, Miwa T, Umeda S, Sawaki K, Tanaka C, Kobayashi D, Hayashi M, Yamada S, Nakayama G, Koike M, Kodera Y. G protein subunit gamma 4 expression has potential for detection, prediction, and therapeutic targeting in liver metastasis of gastric cancer. Br J Cancer. 2021;125:220–8.
Kanda M, Tanaka H, Shimizu D, et al. SYT7 acts as a driver of hepatic metastasis formation of gastric cancer cells. Oncogene. 2018;37:5355–66.
Miwa T, Kanda M, Shimizu D, et al. Hepatic metastasis of gastric cancer is associated with enhanced expression of ethanolamine kinase 2 via the p53-Bcl-2 intrinsic apoptosis pathway. Br J Cancer. 2021;124:1449–60.
Kanda M, Shimizu D, Tanaka H, et al. Synaptotagmin XIII expression and peritoneal metastasis in gastric cancer. Br J Surg. 2018;105:1349–58.
Kanda M, Shimizu D, Tanaka H, et al. Significance of SYT8 for the detection, prediction, and treatment of peritoneal metastasis from gastric cancer. Ann Surg. 2016;267:495–503.
Hafner M, Niepel M, Chung M, et al. Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat Methods. 2016;13:521–7.
Kanda M, Shimizu D, Sawaki K, et al. Therapeutic monoclonal antibody targeting of neuronal pentraxin receptor to control metastasis in gastric cancer. Mol Cancer. 2020;19:131.
Umeda S, Kanda M, Miwa T, et al. Fraser extracellular matrix complex subunit 1 promotes liver metastasis of gastric cancer. Int J Cancer. 2020;146:2865–76.
Sharma S, Ghufran SM, Ghose S, et al. Cytoplasmic vacuolation with endoplasmic reticulum stress directs sorafenib induced non-apoptotic cell death in hepatic stellate cells. Sci Rep. 2021;11:3089.
Kwon OH, Park JL, Baek SJ, et al. Aberrant upregulation of ASCL2 by promoter demethylation promotes the growth and resistance to 5-fluorouracil of gastric cancer cells. Cancer Sci. 2013;104:391–7.
Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15(7):665–74.
Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–7.
Mohrmann R, Dhara M, Bruns D. Complexins: small but capable. Cell Mol Life Sci. 2015;72:4221–35.
Kasai H, Takahashi N, Tokumaru H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol Rev. 2012;92:1915–64.
Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–9.
Proux-Gillardeaux V, Gavard J, Irinopoulou T, et al. Tetanus neurotoxin-mediated cleavage of cellubrevin impairs epithelial cell migration and integrin-dependent cell adhesion. Proc Natl Acad Sci U S A. 2005;102:6362–7.
Pavarotti MA, Tokarz V, Frendo-Cumbo S, et al. Complexin-2 redistributes to the membrane of muscle cells in response to insulin and contributes to GLUT4 translocation. Biochem J. 2021. https://doi.org/10.1042/bcj20200542.
Desiniotis A, Kyprianou N. Significance of talin in cancer progression and metastasis. Int Rev Cell Mol Biol. 2011;289:117–47.
Kim J, Jeong H, Lee Y, et al. HRG-β1-driven ErbB3 signaling induces epithelial-mesenchymal transition in breast cancer cells. BMC Cancer. 2013;13:383.
Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33.
Wu C, Ding H, Wang S, et al. DAXX inhibits cancer stemness and epithelial-mesenchymal transition in gastric cancer. Br J Cancer. 2020;122:1477–85.
Yu L, Peng F, Dong X, et al. Sex-determining region Y chromosome-related high-mobility-group box 10 in cancer: a potential therapeutic target. Front Cell Dev Biol. 2020;8: 564740.
Vander Haar E, Lee SI, Bandhakavi S, et al. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23.
Figueiredo VC, Markworth JF, Cameron-Smith D. Considerations on mTOR regulation at serine 2448: implications for muscle metabolism studies. Cell Mol Life Sci. 2017;74:2537–45.
Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66.
Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–96.
Kanda M, Suh YS, Park DJ, et al. Serum levels of ANOS1 serve as a diagnostic biomarker of gastric cancer: a prospective multicenter observational study. Gastric Cancer. 2020;23:203–11.
Terashima M, Yoshikawa T, Boku N, et al. Current status of perioperative chemotherapy for locally advanced gastric cancer and JCOG perspectives. Jpn J Clin Oncol. 2020;50:528–34.
Yoshida K, Kodera Y, Kochi M, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage iii gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37:1296–304.
Acknowledgements
We thank Taiho Pharmaceutical Co. Ltd for technical support, and advice on this project through discussion on our result.
Funding
This study was funded by Taiho Pharmaceutical Co. Ltd.
Author information
Authors and Affiliations
Contributions
Study concept and design; MK and YK. Acquisition of data; MK, HT, DS, and YK. Analysis and interpretation of data; HT, MK, and YK. Drafting of the manuscript; HT, MK, and YK. Critical revision of the manuscript for important intellectual content; HT, MK, SD, CT, NH, YI, MH, GN, and KY. Statistical analysis; HT and MK. Obtained funding; MK and YK. Technical, or material support; HT, MK, DS, and CT. Study supervision; YK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Brief description: Transcriptomic analysis identified CPLX1 gene as a novel oncogene candidate for gastric cancer. CPLX1 may promote epithelial–mesenchymal transition and evading apoptosis of gastric cancer cells even under a cytotoxic agent, and also be a predictor for recurrence after surgery for UICC Stage II–III gastric cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2022_1884_MOESM4_ESM.pdf
Supplementary file4 Supplemental Fig. S1. Kaplan–Meier plot panel of overall survival, and progression-free survival using external datasets grouped by the median value of each gene expression level to extract 12 candidate genes up-regulated. The left panels present overall survival and the right present recurrence-free survival. Data were retrieved from the GSE62254, GSE15459, GSE22377, GSE62254, GSE29272, and GSE38749, of which UICC Stages were limited to II–III. Supplemental Fig. S2. (a) Conventional immunoblot assay detecting CPLX1 expression of KATOIII cells. (b) Whole picture of digital immunoblot of seven gastric cancer cells (two left panels) and (c) of cells transfected with siRNAs and overexpression vector compared with control siRNA and empty vector, respectively. (d) CPLX1-knockdown efficiencies of N87, OCUM1, and NUGC2 cells. (e) Proliferation assay comparing KATOIII cells, KATOIII cells transfected with siControl, and siCPLX1, and comparing NUGC2 cells transfected with siControl and siCPLX1. Supplemental Fig. S3. (a) Apoptotic cell detection by staining with annexin-V (See Fig. 2e). Phase-contrast and fluorescence images were merged to be presented. (b) Proportions of annexin-V positive stained KATOIII cells untreated, and transfected with siControl and siCPLX1. (c) Drug sensitivity tests to fluorouracil (5-FU) on seven gastric cancer (GC) cell lines. GR values indicate values calculated by normalized growth rate inhibition (GR) metrics. (d) Scatter plot between CPLX1 mRNA expression (CPLX1/GAPDH) and area under the dose–response curves (AUC) to 5-FU of seven GC cell lines. A correlation was tested with the Spearman test. (e) PCR-based EMT-related 84 genes profiling to test co-expression with CPLX1 among 14 GC cell lines. The significances of coefficients were represented by rho (ρ) values. In this panel, other genes generally considered to be related to epithelial–mesenchymal transition (EMT) were presented than the genes presented picked out in Fig. 3. (f) Whole raw pictures of the sandwich ELISA array comparing parental KATOIII cells, that mediated by siControl, and siCPLX1. (g) The whole picture of digital immunoblot of MKN1cells transfected with shCPLX1 compared with the control shRNA (shControl). Supplemental Fig. S4. (a) Histogram of CPLX1 mRNA expression values of 180 samples. The upper quantile value of the present cohort was 0.00682 and indicated by the vertical line. (b) Representative immunohistochemistry of gastric cancer tissue with weak CPLX1 expression and adjacent gastric tissue. (PDF 59442 KB)
Rights and permissions
About this article
Cite this article
Tanaka, H., Kanda, M., Shimizu, D. et al. Transcriptomic profiling on localized gastric cancer identified CPLX1 as a gene promoting malignant phenotype of gastric cancer and a predictor of recurrence after surgery and subsequent chemotherapy. J Gastroenterol 57, 640–653 (2022). https://doi.org/10.1007/s00535-022-01884-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-022-01884-6