Abstract
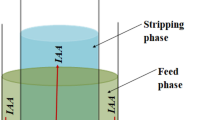
In this study, the removal of nitrate ions from aqueous solutions with liquid membrane technique has been investigated for different organic solvent types in which solubilized tetradecyl trimethyl ammonium bromide (TDTMABr) as carrier. n-butyl alcohol, chloroform, and mixture of chloroform + n-hexane (n-hexane 85% + chloroform 15%) were used as organic solvent. Kinetic parameters (k 1d, k 2m, k 2a, t max, R maxm , J maxm , J maxa ) were calculated from obtained data. time R a values of mixture, butyl alcohol, and chloroform are 0.81, 0.78, and 0.55, respectively. Similarly R d, R m, and t max values of the mixture equal to 0.14, 0.04, and 87.92 min, respectively. This behavior of the system shows the organic solvent type is an effective parameter on separation yield. It can be concluded that the mixture is the most effective organic solvent type among the investigated ones, because liquid membrane systems should be operated within the range of having the R m, R d, and t max values are minimum while R a values are maximum.






Similar content being viewed by others
Abbreviations
- C a :
-
nitrate ion concentration in acceptor phase (mol l−1, M)
- C carrier :
-
carrier concentration (mol l−1, M)
- C do :
-
nitrate concentration in the donor phase at t = 0 moment (mol l−1, M)
- C m :
-
nitrate concentration in organic (membrane) phase (mol l−1, M)
- \(D_{{\rm QNO}_{3}}\) :
-
mean diffusion coefficient of the complex in the membrane of thickness l (m2 s−1)
- J maxa :
-
maximum value of membrane exit flux (min−1)
- J maxd :
-
maximum value of membrane entrance flux (min−1)
- k 1 :
-
membrane entrance rate constant (min−1)
- k 2 :
-
membrane exit rate constant (min−1)
- k 1d :
-
membrane entrance or leak to membrane rate constant (min−1)
- k 2a :
-
acceptor phase entrance rate constant (min−1)
- k 2m :
-
membrane phase exit rate constant (min−1)
- K :
-
equilibrium constant (–)
- R :
-
reduced nitrate ion concentration (–)
- R a :
-
reduced nitrate ion concentration in acceptor phase (–)
- R d :
-
reduced nitrate ion concentration in donor phase (–)
- R m :
-
reduced nitrate ion concentration in membrane phase (–)
- R maxm :
-
maximum reduced nitrate concentration of membrane phase (–)
- S d/m :
-
interface surface of donor and membrane phases (cm2)
- S a/m :
-
interface surface of acceptor and membrane phases (cm2)
- t :
-
time (min)
- t inf :
-
time corresponding to inflection point of the function (min)
- t imax :
-
time which nitrate concentration reaches maximum (min)
- l i :
-
distance between phases (cm)
References
APHA, AWWA, WPCF (1985) Standard Methods for Water and Wastewater Examination, 16th edn. New york
Bogardi I, Kuzelka RD (eds) (1991) Nitrate contamination: exposure, consequence and control. Springer, Berlin Heidelberg New York
Demircioğlu N, Levent M, Kobya M, Topçu N (2000) The effects of stirring speed on coupled transport of nitrite ions through liquid membranes, chemical and biochemical. Eng Q 14(4):109
Demircioğlu N, Topçu N, Levent M, Kobya M, Kocadağıstan E (2000) The effects of stirring speed on coupled transport of nitrate ions through liquid membranes. Bioprocess Eng 22:309
Demircioğlu, Levent M, Kobya M, Topçu N (2000) Kinetic analysis of coupled transport of nitrite ions through liquid membranes at different temperatures. Filtration Sep 37(3):51
Ensafi AA, Eskandari H (2001) Efficient and selective extraction of iodide through a liquid membrane. Microchem J 69(1):45–50
Kobya M, Topcu N, Demircioglu N (1997) Kinetic analysis of coupled transport of thiocyanate ions through liquid membranes at different temperatures. J Membr Sci 130(1–2):7–15
Kobya M, Demirbaş E, Demircioglu N, Yildirim Y, Yildiz YŞ (2004) Effect of carrier type on coupled transport kinetics of thiocynate ions through liquid membranes. Desalination 160:253–262
Koparal AS, Öğütveren ÜB (2002) Removal of nitrate from water by electroreduction and electrocoagulation. J Hazard Mater B89:83–94
Loiacono O, Drioli E, Molinari R (1986) Metal-ion separation and concentration with supported liquid membranes. J Membr Sci 28(2):123–138
Molnar WJ, Wang CP, Evans DF, Cussler EL (1978) Liquid membranes for concentrating anions using a hydroxide flux. J Membr Sci 4(1):129–140
Mulder M (1990) Basic principles of membrane technology. Kluwer, Netherlands
Neplenbroek AM, Bargeman D, Smolders CA (1992) Nitrate removal using supported liquid membranes–transport mechanism. J Membr Sci 67(2–3):107–119
Noble RD, Way JD (1989) Liquid membrane technology, an overview. In: Liquid membranes theory and applications ACS symposium, Ser. No:347, American Chemical Society, Washington DC
Tchobanoglous G, Burton FL (1991) (Reviewers), Biological unit processes, wastewater engineering treatment, disposal and reuse, 3rd edn. McGraw-Hill, Singapore, pp 359–444
Ulbrich M, Marr R (1994) Aqueous liquid membranes—state of the art. Int Chem Eng 34:277
Van der Hoek JP, Van der Hoek WF, Klapwijk A (1988) Nitrate removal from ground water-use of a nitrate selective resin and a low concentrated regenerant water. Air Soil Pollut 37:41–53
Winston WS, Sirkkar KK (1992) Membrane handbook. Chapman and Hall, New York
Yıldız YŞ, İrdemez Ş, Demircioğlu N (2001) Kinetic analysis of coupled transport of nitrate İons through liquid membranes at different temperatures. Fresenius Environ Bull 10(12):869–872
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
İrdemez, Ş., Topçu, N., Yıldız, Y.Ş. et al. Effect of organic solvent type on the removal of nitrate ion using liquid membrane technique. Stoch Environ Res Ris Assess 21, 175–181 (2006). https://doi.org/10.1007/s00477-006-0054-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00477-006-0054-5