Abstract
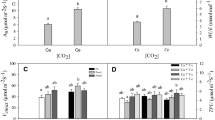
The effects of doubled ambient [CO2] and different temperature levels on young Pinus sylvestris growing in phytotron chambers were studied. Five chambers were supplied with ~380 (‘ambient air’) and five with ~700 μmol mol−1 CO2 (‘elevated [CO2]’). Temperature levels in the chambers ranged in increment steps of 2°C from −4°C to +4°C relative to the long-term monthly (day and night) average air temperature levels in Berlin–Dahlem. Substrate was medium fertile; soil moisture and air humidity were kept constant. After three vegetation periods twigs and stems were harvested, weighed, homogenized, and analyzed chemically. There was no significant temperature effect on wood mass accumulation, clearest positive [CO2] effect occurred in the youngest twigs. In total, wood mass increased by 28.5% at doubled ambient [CO2]. N-contents (percentage) decreased at elevated [CO2] in the uppermost stem sections and not in twig wood causing wider C/N ratios in total. In response to elevated temperature, N-contents decreased slightly in twigs (~0.3%). Traces of free glucose, fructose and sucrose, which decreased from the top to the bottom, were found in stem wood, in contrast to traces of starch that increased from the top to the bottom. In response to elevated [CO2] only a little more (0.05%) was accumulated in the top shoot and in tendency; glucose, fructose, and sucrose contents were lower at the bottom of stems as compared to the control. There was no obvious response of these non-structural carbohydrates to elevated temperature except for starch that decreased to half of the content from the lowest to the highest temperature level. Among the hemicellulose compounds, rhamnose and arabinose declined from the top shoot to the bottom of stem, whereas 4-O-methyl-d-glucuronic-acid, mannose, and xylose increased. Contents (percentage) of galactose remained approximately stable along the stem. The clearest positive effect of elevated [CO2] along the whole stem was found for mannose with differences of 0.6–0.3%. In contrast to rhamnose and arabinose that showed a negative response to elevated [CO2], mannose was reduced towards the uppermost stem sections. The 4-O-methyl-d-glucuronic-acid was slightly lowered at the bottom, and galactose and xylose showed no [CO2] response. The only hemicellulose compound which reacted to temperature elevation was galactose. It increased slightly (~0.1% per 1°C). Cellulose and lignin (Klason) behaved oppositely: cellulose increased and lignin decreased from the top to the bottom. These structural components behaved reversely also in response to elevated [CO2]. In stem parts above the bottom section, cellulose content was slightly higher at elevated [CO2], and lignin content was slightly lower at the bottom. Lignin reacted to temperature elevation by a very slight increase on the average (~0.1% per one 1°C). Cellulose, however, decreased by ~0.2% per 1°C temperature elevation. The importance of persistent sinks of carbon in woody plant parts is discussed in respect to the greenhouse effect.








Similar content being viewed by others
References
Ashworth EN, Stirm VE, Volenec JJ (1993) Seasonal variations in soluble sugars and starch within woody stems of Cornus sericea L. Tree Physiol 13:379–388
Atwell BJ, Henery ML, Whitehead D (2003) Sapwood development in Pinus radiata trees grown for three years at ambient and elevated carbon dioxide partial pressures. Tree Physiol 23:13–21
Barbaroux CN, Bréda N (2002) Contrasting distribution and seasonal dynamics of tree carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol 22:1201–1210
Barton CVM, Jarvis PG (1999) Growth response of branches of Picea sitchensis to four years exposure to elevated atmospheric carbon dioxide concentration. New Phytol 144:233–243
Bauch J, Koch G, Puls J, Schwarz T, Voiß S (2006) Wood characteristics of Podocarpus oleifolius var. macrostachys (Parl.) Buchholz and Gray, native to Costa Rica: their significance for wood utilization. Wood Sci Technol 40:26–38
Berman ME, Dejong TM (2003) Seasonal patterns of vegetative growth and competition with reproductive sinks in peach (Prunus persica). J Hortic Sci Biotechnol 78:303–309
Bollmark L, Sennerby-Forsse L, Ericsson T (1999) Seasonal dynamics and effects of nitrogen supply rate on nitrogen and carbohydrate reserves in cutting-derived Salix viminalis plants. Can J For Res 29:85–94
Bruhn D, Leverenz JW, Saxe H (2000) Effects of tree size and temperature on relative growth rate and its components of Fagus sylvatica seedlings exposed to two partial pressures of atmospheric [CO2]. New Phytol 146:415–425
Claus I (2005) Eignung des MEA-Verfahrens zur Herstellung von Chemie- und Papierzellstoffen. Dissertation, Universität Hamburg, pp 50–60
Davey PA, Olcer H, Zakhleniuk O, Bernacchi CJ, Calfapietra C, Long SP, Raines CA (2006) Can fast-growing plantation trees escape biochemical down-regulation of photosynthesis when grown throughout their complete production cycle in the open air under elevated carbon dioxide? Plant Cell Environ 29:1235–1244
DeLucia EH, Callaway RM, Schlesinger WH (1994) Offsetting changes in biomass allocation and photosynthesis in Ponderosa pine (Pinus ponderosa) in response to climate change. Tree Physiol 14:669–677
Domisch T, Finér L, Lehto T (2001) Effects of soil temperature on biomass and carbohydrate allocation in Scots pine (Pinus sylvestris) seedlings at the beginning of the growing season. Tree Physiol 21:465–472
Druart N, Rodriguez-Buey M, Barro-Gafford G, Sjödin A, Bhalerao R, Hurry V (2006) Molecular targets of elevated [CO2] in leaves and stems of Populus deltoides: implications for future growth and carbon sequestration. Funct Plant Biol 33:121–131
Egger B, Einig W, Schlereth A, Wallenda T, Magel E, Loewe A, Hampp R (1996) Carbohydrate metabolism in one- and two-year-old spruce needles, and stem carbohydrates from three months before until three months after bud break. Physiol Plant 96:91–100
Fengel D, Wegener G (1984) Wood-chemistry, ultrastructure, reactions. Walter de Gruyter, Berlin, pp 1–613
Fischer C, Höll W (1992) Food reserves of Scots pine (Pinus sylvestris L.) II. Sesonal changes and radial distribution of carbohydrate and fat reserves in pine wood. Trees 6:147–155
Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin K, Delmer DP (2001) Carbon partitioning to cellulose synthesis. Plant Mol Biol 47:29–51
Hawes M, Celog R, Price I, Wen F, Ebolo E (2007) Galactose from the legume root cap: structure, signal, toxin, trigger? Comp Biochem Physiol Part A 146:267
Hobbie EA, Gregg J, Olszyk DM, Rygiewicz PT, Tingey DT (2002) Effects of climate change on labile and structural carbon in Douglas fir needles as estimated by delta C-13 and C-area measurements. Glob Change Biol 8:1072–1084
Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26:1067–1081
Höll W (2000) Distribution, fluctuation and metabolism of food reserves in the wood of trees. In: Savidge R, Barnett J, Napier R (eds) Cell & molecular biology of wood formation. BIOS Scientific Publisher Ltd, Oxford, pp 347–362
Hu WJ, Harding SA, Lung J, Popko JL, Ralph J, Stokke DD, Tsai CJ, Chiang VL (1999) Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat Biotechnol 17:808–812
Irbe I, Andersons B, Chirkova J, Kallavus U, Andersone I, Faix O (2006) On the changes of pinewood (Pinus sylvestris L.) Chemical composition and ultrastructure during the attack by brown-red fungi Postia placenta and Coniophora puteana. Int Biodeterior Biodegradation 57:99–106
Jung HG, Mertens DR, Payne AJ (1997) Correlation of acid detergent lignin and Klason lignin with digestibility of forage dry matter and neutral detergent fibre. J Diary Sci 80:1622–1628
Kaakinen S, Kostiainen K, Ek F, Saranpää P, Kubiske ME, Sober J, Karnosky DF, Vapaavuori E (2004) Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Glob Change Biol 10:1513–1525
Kaakinen S, Saranpää P, Vapaavuori E (2007) Effects of growth differences due to geographic location and N-fertilisation on wood chemistry of Norway spruce. Trees 21:131–139
Kilpeläinen A, Peltola H, Ryyppö A, Sauvala K, Laitinen K, Kellomäki S (2003) Wood properties of Scots pines (Pinus sylvestris) grown at elevated temperature and carbon dioxide concentration. Tree Physiol 23:889–897
Kilpeläinen A, Peltola H, Ryyppö A, Kellomäki S (2005) Scots pine responses to elevated temperature and carbon dioxide concentration: growth and wood properties. Tree Physiol 25:75–83
Lamlon SH, Savidge RA (2003) A reassessment of carbon content in wood: variation within and between 41 North American species. Biomass Bioenergy 25:381–388
Liu L, King JS, Giardina CP (2005) Effects of elevated concentrations of atmospheric CO2 and tropospheric O3 on leaf litter production and chemistry in trembling aspen and paper birch communities. Tree Physiol 25:1511–1522
Magel E, Abdel-Latif A, Hampp R (2001) Non-structural carbohydrates and catalytic activities of sucrose metabolizing enzymes in trunks of two Juglans species and their role in heartwood formation. Holzforschung 55:135–145
Mandre M, Pärn H, Ots K (2006) Short-term effects of wood ash on the soil and the lignin concentration and growth of Pinus sylvestris L. For Ecol Manag 223:349–357
Oksanen E, Riikonen J, Kaakinen S, Holopainen T, Vapaavuori E (2005) Structural characteristics and chemical composition of birch (Betula pendula) leaves modified by increasing CO2 and ozone. Glob Change Biol 11:732–748
Overdieck D (1993) Elevated CO2 and the mineral content of herbaceous and woody plants. Vegetatio 104(105):403–411
Overdieck D, Reid C, Srain BR (1988) The effects of preindustrial and future CO2 concentrations on growth, dry matter production and the C/N relationship in plants at low nutrient supply: Vigna unguiculata (Cowpea), Abelmoschus esculentus (Okra) and Raphanus sativus (Radish). Angew Botanik 62:119–134
Peltola H, Kilpeläinen A, Kellomäki S (2002) Diameter growth of Scots pine (Pinus sylvestris) trees grown at elevated temperature and carbon dioxide concentration under boreal conditions. Tree Physiol 22:963–972
Pereira H, Graça J, Rodriguez JC (2003) Wood chemistry in relation to quality. In: Barnett JR, Jeronimidis G (eds) Wood quality and its biological basis. Blackwell Publishing, CRC press, pp 5386
Piispanen R, Saranpäa P (2001) Variation of non-structural carbohydrates in silver birch (Betula pendula Roth) wood. Trees 15:444–451
Puls J (1982) Chemical analysis of lignocellulose residues. In: Strub A et al (eds) Energy from biomass. Applied Science Publishers, London, pp 863–867
Runion GB, Entry JA, Prior SA, Mitchell RJ, Rogers HH (1999) Tissue chemistry and carbon allocation in seedlings of Pinus palustris subjected to elevated CO2 and water stress. Tree Physiol 19:329–335
Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22:583–621
Teleman A, Nordström M, Tenkanen M, Jacobs A, Dahlmann O (2003) Isolation and characterization of O-acetylated glucomannan from aspen and birch wood. Carbohydr Res 338:525–534
Terziev N, Boutelje J, Larsson K (1997) Seasonal fluctuations of low-molecular-weight sugars, starch and nitrogen in sapwood of Pinus sylvestris L. Scand J For Res 12:216–224
Tjoelker MG, Oleksyn J, Reich PB (1999) Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Glob Change Biol 49:679–691
Toivanen TJ, Alén R (2006) Variations in the chemical composition within pine (Pinus sylvestris) trunks determined by diffuse reflectance infrared spectroscopy and chemometrics. Cellulose 13:53–61
Turnbull H, Tissue DT, Griffin KL, Rogers GND, Whitehead D (1998) Photosynthetic acclimation to long-term exposure to elevated CO2 concentration in Pinus radiata D. Don. is related to age of needles. Plant Cell Environ 21:1019–1028
Usami T, Lee J, Oikawa T (2001) Interactive effects of increased temperature and CO2 on the growth of Quercus myrsinaefolia saplings. Plant Cell Environ 24:1007–1019
Willför S, Holmbom B (2004) Isolation and characterisation of water soluble polysaccharides from Norway spruce and Scots pine. Wood Sci Technol 38:173–179
Wu L, Chandrashekar PJ, Chiang VL (2000) A xylem-specific cellulose synthase gene from aspen (Populus tremuloides) is responsive to mechanical stress. Plant J 22:495–502
Yeh TF, Goldfarb B, Chang H, Peszlen I, Braun JL, Kadla JF (2005) Comparison of morphological and chemical properties between juvenile wood and compression wood of Loblolly pine. Holzforschung 59:669–674
Ziche D, Overdieck D (2004) CO2 and temperature effects on growth, biomass production, and stem wood anatomy of juvenile Scots pine (Pinus sylvestris L.). J Appl Bot Ange Bot 78:120–132
Acknowledgments
The research was funded by the 5th Framework programme of the European Commission through the MEFYQUE research contract QLKS-CT-2004. Special thanks are addressed to Dr. J. Puls from the Institute of Wood Chemistry and Chemical Technology of Wood/Research Centre for Forestry and Wood-Economy in Hamburg, Germany. He contributed know-how, use of instruments and many analyses especially to the part about hemicellulose components, cellulose and lignin. Language was ‘polished’ by Sally Johnson.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Lüttge.
Rights and permissions
About this article
Cite this article
Overdieck, D., Fenselau, K. Elevated CO2 concentration and temperature effects on the partitioning of chemical components along juvenile Scots pine stems (Pinus sylvestris L.). Trees 23, 771–786 (2009). https://doi.org/10.1007/s00468-009-0319-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-009-0319-y