Abstract
Lymphatic filariasis (LF) is an important neglected parasitic disease according to the World Health Organization. In this study, we aimed to determine the prevalence of human LF in Asia using a systematic review and meta-analysis approach. Records from 1990 to 2018 in reputable databases including PubMed, Science Direct, Embase, and Cochrane Library were searched using a panel of related keywords. All 48 countries of Asia were searched one by one in combination with the keywords. In all, 41,742 cases identified in this study were included in the analysis. According to our findings, the pooled prevalence of LF in Asia was estimated at 3% (95% CI: [1.7, 5.2]). There was no major trend in the cumulative prevalence of LF over time. Some countries in Asia including China, Japan, Vietnam, and South Korea succeeded in eliminating LF as a public health problem, but others still need to monitor the disease. Based on the initiative of the WHO starting in 2000, some countries in Asia succeeded in eliminating LF as a public health problem. Other countries have taken steps to eliminate the disease with variable degrees of success. These efforts might be affected by issues such as climate change.
Similar content being viewed by others
Introduction
The term neglected tropical diseases (NTDs) arose from the recommendation by the Working Group on Monitoring and Evaluation of the Strategic and Technical Advisory Group for NTDs (WHO 2020). Among NTDs, parasitic diseases are prominent, and many studies have been conducted on different aspects of their risks and complications (Torgerson et al. 2014). According to the WHO (WHO 2020), the following diseases are considered NTDs: Chagas disease, dracunculiasis (guinea-worm disease), echinococcosis, foodborne trematodiases, human African trypanosomiasis (sleeping sickness), leishmaniasis, lymphatic filariasis, onchocerciasis (river blindness), schistosomiasis, soil-transmitted helminthiasis, and taeniasis/cysticercosis.
Filariasis is an important parasitic disease caused by roundworms of the Filarioidea superfamily, which are parasites residing in the blood and tissues of humans. In humans, filariasis is caused by Wuchereria bancrofti, Brugia malayi, Loa loa, Onchocerca volvulus, and Dirofilaria spp. Lymphatic filariasis (LF), in which the adult worms are found in the lymphatic system, is considered the most important form of filariasis, and is also known as elephantiasis. It is transmitted by mosquitoes of the genera Culex, Mansonia, and Anopheles (Solgi et al. 2017; WHO 2013).
Nearly 63% of 1.34 billion people worldwide are at risk of LF, and about 50% of the 120 million infected people live in the South-East Asia Region. This region bears approximately 57% of the total global burden of an estimated 5.1 million disability-adjusted life years (DALYs) lost due to LF. Nine countries in this region are endemic for LF. India, Nigeria (in Africa), Bangladesh and Indonesia together account for 70% of LF in the world, although 80 countries are considered endemic for the disease (WHO 2013). In the year 2016, a total of 1189 (587.7 to 2114.9) DALYs (thousands) were reported for all ages (Collaborators 2017).
Countries and areas considered at high risk include central Africa and the Nile delta, Madagascar, Turkey, the Middle East, India, Pakistan, Sri Lanka, Myanmar, Thailand, Malaysia, Vietnam, South Korea, Indonesia, the Philippines, Timor, southern China, Haiti, Dominican Republic, Guyana, French Guinea, and costal Brazil (Hotez and Ehrenberg 2010; Utzinger et al. 2010).
The disease has variable symptoms caused mostly because adult worms occupy and block the lymphatic vessels. Depending on the kind of filariasis, the patient shows a spectrum of signs and symptoms including elephantiasis, lymphedema, hydrocele, chyluria, chylous diarrhea, and chylorrhagia (Addiss 2010; Kabatereine et al. 2010). Some asymptomatic cases, which may become chronic, have also been reported.
Clinical manifestations including episodic attacks of lymphadenitis and lymphangitis (fever, pain in the affected area, tender red streaks) along with fever and malaise are attributed to acute LF, while some cases of acute attacks have been reported for chronic manifestations. The patients show lumps in the subcutaneous tissue, breasts or testicles due to reactions to adult worms or related granulomas (Al-Shaham and Sood 2010).
After the World Health Assembly (WHA) on 1997 and based on Resolution 50.29 to eliminate LF, a Global Programme to Eliminate LF (GPELF) was initiated in 2000 to eliminate LF in 2020. The main strategies were mass drug administration (MDA) using a two-drug combination of diethylcarbamazine (DEC) and albendazole, and a transmission assessment survey (TAS) (WHO 2013).
In this review, we aimed to collect and analyze data concerning the situation of human filariasis in Asian countries by searching publications between 1990 and 2018 recorded in reliable databases.
Methods
Search strategy
The PubMed, Science Direct, Embase, and Cochrane Library databases were searched using a panel of keywords that included, but was not limited to, human filariasis, lymphatic filariasis, Wuchereria bancrofti, Brugia malayi, Brugia timori, prevalence, epidemiology, and all Asia countries in turn (https://www.worldatlas.com/articles/how-many-countries-are-in-asia.html). The countries included in the searches were Afghanistan, Armenia, Azerbaijan, Bahrain, Bangladesh, Bhutan, Brunei, Cambodia, China, Cyprus, East Timor, Georgia, India, Indonesia, Iran, Iraq, Israel, Japan, Jordan, Kazakhstan, Kuwait, Kyrgyzstan, Laos, Lebanon, Malaysia, Maldives, Mongolia, Myanmar, Nepal, North Korea, Oman, Pakistan, Philippines, Qatar, Saudi Arabia, Singapore, South Korea, Sri Lanka, State of Palestine, Syria, Tajikistan, Thailand, Turkey, Turkmenistan, United Arab Emirates, Uzbekistan, Vietnam and Yemen.
We included relevant studies conducted from 1990 to 2018 and published in English. We initially screened studies based on their title and abstract, followed by availability of the full text. If only the abstract was available, information from the study was considered only in the Discussion section. Items such as books and Letters to the Editors were excluded from the study.
The inclusion criteria were (1) conducted between 1990 and 2018, (2) English language, (3) availability of the full text, (4) original or review article as publication type, and (5) involving human patients.
The exclusion criteria were (1) studies in animals only, and (2) case report or letter to the editor as publication type.
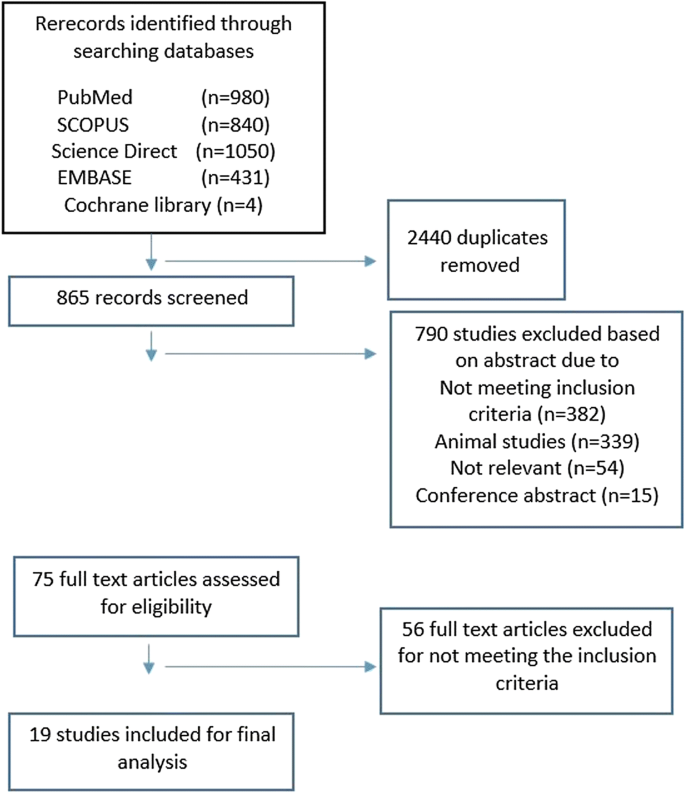
As shown in Fig. 1, a total of 3305 records were initially found in the database searches. After initial screening, 2440 duplicate articles were deleted. The remaining 790 items were then screened to locate relevant content. The remaining 75 articles were then checked to verify that they met the inclusion criteria, and as a result, a total of 19 studies were included in the analysis.
Statistical analysis
The data were analyzed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria.). The pooled prevalence was estimated with its 95% confidence interval (CI). Chi-squared, tau-squared, and I-squared statistics were calculated to assess heterogeneity of the studies. Due to significant heterogeneity, a random effects model was used to estimate the pooled prevalence of filariasis. Publication bias was evaluated using a funnel plot. Results with p values less than 0.05 were considered statistically significant.
Results and discussion
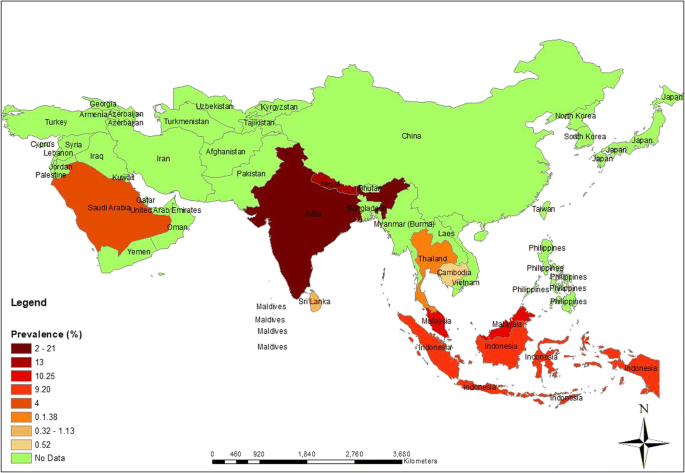
Figure 2 shows the distribution of LF in Asia. The differences in prevalence among countries are discussed in detail below.
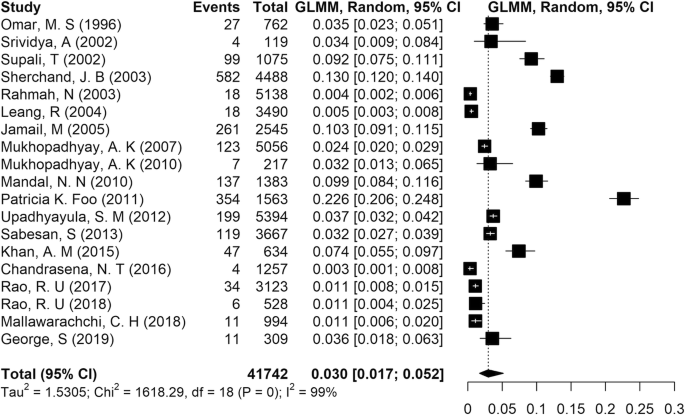
A total of 41,742 cases synthesized in the present study were included in the analysis. According to the results of the present meta-analysis, reported in Fig. 3, the pooled prevalence of LF in Asia was estimated at 3% (95% CI: [1.7, 5.2]). The results of chi-squared, tau-squared and I-squared tests revealed significant heterogeneity among the studies, so a random effects model was used to pool the prevalences from all included studies (tau2 = .53, chi2 = 1618.02, p value < 0.001, I2 = 99%).
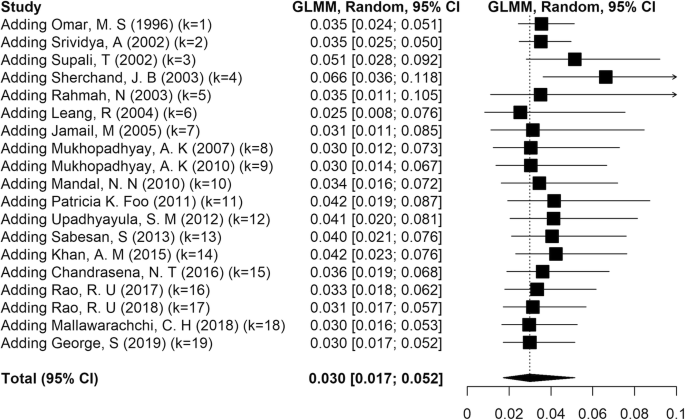
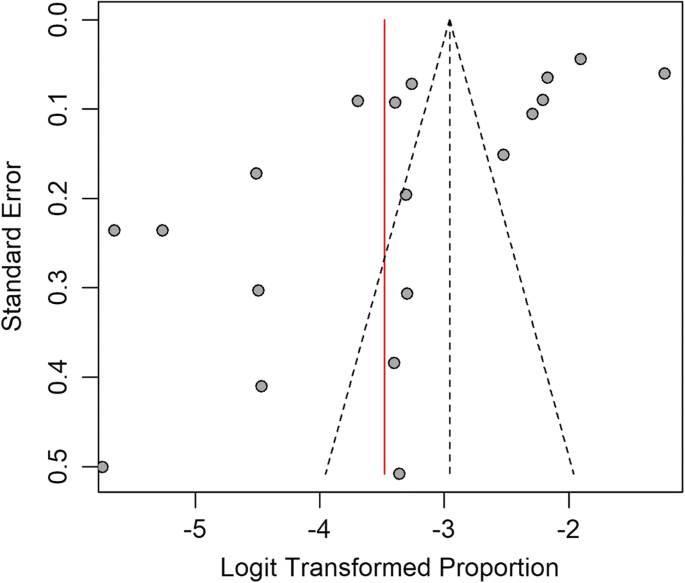
Cumulative meta-analysis was performed to estimate the trend in LF prevalence with time in Asia. The results showed that there was no major trend in cumulative prevalence (Fig. 4). A funnel plot drawn to check for the existence of publication bias in the studies (Fig. 5) showed no bias.
The highest prevalence of infection was reported in India as 21% (Foo et al. 2011), and the lowest rate was from Sri Lanka as 0.32% (Chandrasena et al. 2016). We noted that most studies done in India reported a prevalence of LF between 2.4 and 21% (Foo et al. 2011; George et al. 2019; Khan et al. 2015; Mandal et al. 2010; Mukhopadhyay 2010; Mukhopadhyay et al. 2007; Sabesan et al. 2013; Srividya et al. 2002; Upadhyayula et al. 2012).
Lymphatic filariasis due to Brugia spp. was reported from Indonesia (Supali et al. 2002), Malaysia (Jamail et al. 2005), Sri Lanka (Iddawela et al. 2015; Mallawarachchi et al. 2018), and Thailand (Rahmah et al. 2003). Dirofilaria spp. was reported in humans in Iran (Ashrafi et al. 2010; Ghasemi et al. 2020; Jamshidi et al. 2008; Tavakolizadeh and Mobedi 2009), Israeli (Gutierrez et al. 1995), and Taiwan (Li et al. 2013), although these cases are not considered NTDs. Because the aim of the study was to document the situation of LF only in humans, the many studies that reported animal filariasis in Asia were excluded from the present analysis.
The situation of LF in individual Asian countries is reported below.
India
Lymphatic filariasis has a long history in India dating back to the 6th century B.C. In 1995, the National Filaria Control Programme (NFCP) was started (Agrawal and Sashindran 2006). The disease has been reported in India since 1945 (Ahmad 1945). Unfortunately, there are no detailed data in the literature from that time. A survey conducted in 1981 showed that of 24,946 persons examined for LF, 8–41% had microfilaremia (Rajagopalan et al. 1989).
Many more recent studies from India have been published regarding the prevalence of LF (Foo et al. 2011; George et al. 2019; Khan et al. 2015; Mandal et al. 2010; Mukhopadhyay 2010; Mukhopadhyay et al. 2007; Sabesan et al. 2013; Srividya et al. 2002; Upadhyayula et al. 2012). Bancroftian filariasis was reported in all of these studies. India harbors nearly 40% of 120 million cases of infection with LF in the world (George et al. 2019). The DALYs lost in India due to FL has been reported as 2.06 million, resulting in an annual wage loss of US $811 million (Ottesen 2000). The six states regarded as having high endemicity in India are Uttar Pradesh, Bihar, Jharkhand, Orissa, Kerala, and Gujarat (Raju et al. 2010). The global LF program is currently being conducted with the main targets of eliminating LF and interrupting transmission using MDA (Ottesen 2000). The drugs DEC and albendazole are used as part of the program to support its aims. Accordingly, they may reduce the microfilaremia more than 95% after 2 annul rounds (Ottesen 2000).
Malaysia
In Malaysia, LF has been reported with two agents: W. bancrofti and B. malayi (Al-Abd et al. 2014). The first report of LF we noted in the literature dates to 1968, but no details were available (Yap et al. 1968). The earliest report we could find described microfilaria as sub-periodic B. malayi in 82 persons among 1613 people examined in seven villages in Serian District (Rubis et al. 1981). The disease is not widespread in the country, and occurs only in some states of Peninsular Malaysia including Terengganu, Kelantan, Pahang, Selangor, and Johor as well as the very small Sabah and Sarawak regions (Al-Abd et al. 2014). In Malaysia, an MDA program is being conducted with the two drugs noted earlier for India. The program covered all endemic areas from 2004 to 2008. It appears that the program has not been completely successful and requires additional efforts to reach the WHO goals (Al-Abd et al. 2014; Noordin et al. 2017). In a study by Rahmah et al. (Rahmah et al. 2003) with B. malayi recombinant antigen for ELISA testing, among 5138 children in Malaysia, 0.35% showed seropositivity. Positive cases included 13 boys and 5 girls between 7 and 12 years old.
Sri Lanka
Lymphatic filariasis has been reported from Sri Lanka for hundreds of years, with high endemicity (Rao et al. 2018). The earliest documented report found microfilaremia in 19.1% of houses examined for LF in Ceylon (Abdulcader et al. 1966). The country implemented an MDA program with DEC and albendazole from 2002 to 2006 in all endemic parts of the country. A TAS was conducted in endemic areas in 2013 (WHO 2014) to determine the prevalence of filarial antigenemia in young schoolchildren, and found a prevalence of less than 2% with 95% certainty (Chu et al. 2013). Rao et al. believed this method was not sensitive to detect ongoing transmission of W. bancrofti in many areas in Sri Lanka (Rao et al. 2018). They reported high rates of circulating filarial antigenemia (3%, confidence interval [CI]: 1.8–4.9) and microfilaremia (1%, CI: 0.5–2.5%), and noted that “circulating filarial antigenemia rates were 2.8-fold higher in males than females”. Anti-filarial antibodies were detected in young children at a prevalence of 5.7% (Rao et al. 2018). According to the WHO, although Sri Lanka could eliminate LF in 2016, surveillance efforts and interventions should be continued to monitor the problem in endemic areas (WHO 2016). It is believed that according to antigen prevalence data, adult males account for most persistent filarial infections in Sri Lanka (Irvine et al. 2018; Rao et al. 2017, 2018).
Indonesia
The reported cases of LF in this country were 9.2% and 1.98% (Ginandjar et al. 2018; Supali et al. 2002). In 1980, the prevalence of microfilaremia was 19.5%, but it had been reduced to 4.7% by 2014 (Lee and Ryu 2019). The Indonesian Ministry of Health planned an MDA program in 2015 to monitor LF in 106 endemic districts. Based on further verifications, there was little success in monitoring the disease, such that 29 provinces continued to have problem in Eastern Indonesia in 2016 (Lee and Ryu 2019; Wibawa and Satoto 2016). A known endemic district is Pekalongan, where 62 cases with chronic LF were reported. There is a risk that the disease will spread if it is not controlled (Ginandjar et al. 2018). In a study of elementary schoolchildren in Indonesia in 2015, the prevalence was 1.98% and the agent was W. bancrofti. Although most infected students were older ones and males, no significant differences were reported (Ginandjar et al. 2018). In another study (Supali et al. 2002), both bancroftian filariasis and B. timori were reported in separate districts but no mixed infections were detected. Among 586 cases studied for B. timori, 27% showed microfilaremia. Males showed more infection than females. Regarding clinical manifestations, 13% of cases showed lymphedema in the legs, but no hydrocele or genital lymphedema were reported.
Nepal
Another country in Asia, which is listed by the WHO as an endemic country for LF, is Nepal (Sherchand et al. 2003). The only agent of LF in Nepal is W. bancrofti, transmitted by Culex quinquefasciatus. Our survey of the literature disclosed no data regarding the history of LF in Nepal in the past, and identified only some reports which testify to the endemic nature of LF (Pradhan et al. 1998; Rana Krishna 2003). According to the Department of Health Services (DoHS) (2020) and the Epidemiology and Disease Control Division Teku (2018) and based on a survey from 2001 to 2012, the prevalence of LF was reported to range between 1 and 39% with average of 13%. It was also reported that 25 million people are at risk of LF in 61 out of 75 districts in Nepal. All these 61 endemic districts have received six rounds of MDA. Since 2018, 14 districts have been considered nonendemic. The MDA program was stopped in 36 districts in light of a successful TAS, but 25 districts were scheduled for MDA in 2019. Morbidity data recorded during the MDA from 61 districts showed a total of 28,529 cases of LF, among which most (19,907) were hydrocele, 5704 were elephantiasis, and cases 2918 involved hand and breast swelling and other LF manifestations (Epidemiology and Disease Control Division Teku 2018). At present, many health workers have learned to manage the disease and teach people how to prevent, manage and cure the LF. A referral system is available, along with treatment free of charge for infected people. The government of Nepal has implemented six rounds of MDA, instead of the five rounds implemented in other countries, to ensure the elimination of LF as a public health problem (Epidemiology and Disease Control Division Teku 2018). These rounds used a single dose of albendazole plus DEC as the baseline for MDA. A study of 4488 people in Nepal showed 13% seropositivity for LF. A higher rate of infection was reported among males (57.4%), but the difference between males and females was not significant. In addition, seropositivity was highest in the group of people 46 to 50 years old, with the lowest rate in the 36-to-40-year-old age group, but here again the difference was not significant (Sherchand et al. 2003).
Thailand
A noteworthy feature of Thailand is this country’s extensive border with Myanmar. It has been documented that there is no significant rate of LF in Thai people, and the disease has been reported only in one southern province. Regarding the history of LF in Thailand, an old study reported that among 4112 persons examined in many villages, 863 were positive for microfilariae, of whom 215 showed filarial disease (Iyengar 1953). Currently, most cases of LF are reported in immigrants (Nithikathkul et al. 2006; Rojanapanus et al. 2019). Many immigrants reside in Thailand, so many studies have focused on this population group. In a long-term study from 2002 to 2017, LF was investigated in 23,477 immigrants. The results showed that 0.7% (range 0.1 to 2.7%) of them were seropositive (Rojanapanus et al. 2019). In contrast, during the same period, no positive cases were detected among Thai people in nearby areas. In another study of 2462 people of Thai origin, 1.38% were positive for B. malayi microfilariae The highest prevalence (4.69%) was reported in the 45-to-60-year-old group , and the lowest rate (0.37%) in the less than 15-year-old age group. The rate of infection was threefold as high in males as in females (Triteeraprapab et al. 2001). Toothong et al. evaluated the efficacy of MDA in 2015, and found that 75% of immigrants received DEC, which was below the standard. Barriers to receiving DEC were lack of official documents, unemployed status, daily employment, short-term immigrant status, and living in a fishery area for immigrants (Toothong et al. 2015). Currently, an LF surveillance program is conducted by the government every 2 years, targeted especially to immigrants. The perspectives seem promising in terms of eliminating LF as a public health problem (Rojanapanus et al. 2019).
Saudi Arabia
In Saudi Arabia, there are no significant concern at present regarding LF. Most cases have been reported among foreigner workers or as case reports (El-Moamly et al. 2012; Haleem et al. 2002; Omar 1996). In a study conducted by El-Moamly et al., among 647 foreigner workers from countries endemic for LF, 32 (5%) were positive for W. bancrofti microfilaremia according to membrane filtration and microscopy, 142 (22%) according to ELISA, and 128 (20%) according to an immunochromatographic test (ICT) (El-Moamly et al. 2012). Thus, FL in this country mostly a potential problem because of the huge number of foreigner workers, but not in people of Saudi origin. Another study of foreigner workers (n = 762) to determine the rate of LF showed that 3.5% of the participants were positive. A total of 259 Indian males had an mf density of 6.0/20 mm3 of blood. To date, only W. bancrofti has been detected as the only causal agent of LF, and in 1996, it was first suggested that Culex pipiens mosquitoes might be a potential vector for introduced LF in this country (Omar 1996). These results show that people of Saudi origin are at risk of the disease and should be aware of the risk. In one of the few studies of LF in people of Saudi origin from 1981 to 2001 at a military hospital, three cases were reported. They included a 68-year-old woman, a 78-year-old woman, and a 32-year-old man, all of Saudi origin. The authors warned that indigenous filariasis is present in Saudi Arabia, and that this disease should be considered in patients who show compatible signs or symptoms on physical examination (Haleem et al. 2002).
Cambodia
The earliest report of existing LF in this country dates back to 1956, when microfilaria was discovered in mosquitoes (Urbani 1997). The first case of LF was reported in 1995, followed by other positive cases in a single village (Leang et al. 2004). Cambodia contains both B. malayi and W. bancrofti microfilariae (Khieu et al. 2018; Leang et al. 2004). During a survey in 2004 by Leang et al., LF was investigated in 3490 people with a compendium of methods. The results showed that 0.52% were infected with filariasis according to the WB ICT card test, and that 0.23% were infected according to night blood examination (Leang et al. 2004). Both B. malayi and W. bancrofti were detected as the agents. The Cambodian Ministry of Health inaugurated an initiative in 2003 to control and eliminate NTDs including LF, by 2015. In overall terms this target was achieved, thanks in part to positive collaboration among the responsible organizations. In addition, MDA covered more than 70% of the country in five consecutive rounds from 2005 to 2009. This achievement was possible thanks to a compendium of training, allocation of two reference hospitals, MDA, screening tests, and other measures (Khieu et al. 2018). It was also announced that antigenemia in schoolchildren decreased from 1 to 0% during the years 2010–2013 and 2015. Together, these achievements led the WHO to announce that Cambodia was free of LF in June 2016 (Khieu et al. 2018).
China
China is among the countries which has not only certified the elimination of LF as a public health problem, but has also has entered the post-elimination survivable phase (Fang and Zhang 2019). Previously, both W. bancrofti and B. malayi infections were prevalent in this country. In the 1980s, 31 million cases of LF were estimated including 22 million as bancroftian filariasis and 9 million as malayan filariasis (Anonymous 1991). Sixteen provinces were involved then, including 864 counties and cities. The nature of filariasis in these areas was bancroftian (n = 463), malayan (n = 217), and mixed infections (n = 184) (Sun and Chen 1992). In 1995, elimination was first announced in Guanxi, and the province to eliminate the disease was Anhui in 2006. Thereafter, the WHO announced that China was the first country in the world to officially succeed in eliminating LF as a public health problem (De-Jian et al. 2013). Some strong points helped China to combat the disease, e.g., an emphasis on control of infectious sources, three rounds of DEC, and establishing a threshold for LF transmission interruption (Fang and Zhang 2019). Although the present situation is ideal, the government plans to appraise the TSA in some previously endemic areas during the next 2 years.
Other countries
Vietnam is among the countries that, according to the WHO, has successfully eliminated LF (Cane 2020; Serrano et al. 2020). Reports of the disease in this country date from the 1900s (Meyrowitsch et al. 1998). A study in 1998 surveyed 135,000 people from 24 provinces, and found a prevalence of microfilaremia, attributed primarily to B. malayi, ranging from 0.9 to 5.5% (Meyrowitsch et al. 1998).
The Philippines reported LF caused by both W. bancrofti and B. malayi from 1951 onwards. With the establishment of the National Filariasis Control Programme in 1963, the government tried to identify endemic areas (Leonardo et al. 2020). Forty-six provinces (of a total of 81) had cases of LF. It is reported that the disease was more prevalent in adults than children, and in males compared to females (Kron et al. 2000; Leonardo et al. 2020). Like other countries at risk for LF, the Philippines started to combat the disease using a compendium of MDA, morbidity management and prophylaxis from 2000. Before that, 40 million people were at risk of infection, and in 1998 the national prevalence rate of LF was 9.7 cases per 1000 population (Galvez Tan 2003; Kron et al. 2000; Rubite 2018). Five rounds of MDA were implemented in 38 endemic provinces. Following on from this achievement, the government hopes to eliminate LF in the country by 2020.
The Republic of Korea is documented to be free of LF (Cheun et al. 2009; Cheun et al. 2017). Official reports of LF in this country date back to 1927. Although W. bancrofti was initially identified as the culprit species, later B. malayi was determined to be the correct culprit (Senoo 1943). In the 1950s, it was reported that 12.1% of the population was positive for microfilaria caused by B. malayi (Senoo and Lincicome 1951). Later studies showed that the infection was remained present in Korea (Cheun et al. 2017); however, at present, the country is documented to be free of LF. This achievement was made possible by government initiatives including the installation of mosquito nets and sanitation in houses (Cheun et al. 2009). During a follow-up study conducted by Cheun et al., 83 patients with an earlier diagnosis of LF were surveyed, and no cases were detected in many of them, although 31 patients could not be traced for different reasons (Cheun et al. 2017).
Bangladesh still has not eliminated LF but has made significant progress towards this goal (Karim et al. 2019; Shamsuzzaman et al. 2017). An MDA program was successfully implemented in the country, when it was assumed that 70 million people were at risk of LF. The species involved was identified as W. bancrofti transmitted by Culex sp. In 2001, 34 out of 64 districts were endemic for microfilaremia, at a rate of 1 to 19% (Hafiz et al. 2015; Karim et al. 2019; Shamsuzzaman et al. 2017). Overall, 19 endemic areas successfully completed the MDA program while 15 others were excluded from MDA monitoring because of their low endemicity (WHO 2011). One recent study (Karim et al. 2019) detected 43,678 clinical cases in 19 highly endemic districts, including cases of leg and/or arm lymphedema, hydrocele, female breast lymphedema or genital swelling. It shows that the government should implement additional measures to eliminate the infection. In another study to detect clinical cases of LF in 30 villages of Nilphamari District in Bangladesh, among 1242 participants, 4.4% were found to have LF-related clinical conditions. The most frequent clinical manifestations were hydrocele in males and leg lymphedema in females (Hafiz et al. 2015).
In Myanmar, the prevalence of LF remains high. The species involved is W. bancrofti, transmitted by Culex quinquefasciatus (Aye et al. 2018; de Meillon et al. 1967). In accordance with the WHO, the government of Myanmar decided in 2020 to launch a program to eliminate LF (Research et al. 1998). As a result, it was found that about 41 million people (about 80% of the total population) were infected with LF in 45 of 65 districts. An MDA program was implemented in endemic areas. To appraise the degree of success, two studies were conducted in 2008 and 2014 (Aye et al. 2018; WHO 2004). The results did not show complete elimination, but varying degress of progress were made and in some areas, the outcome was significant. The rate of filarial antigen positivity ranged from 0 to > 25% (Aye et al. 2018). A recent study showed the overall prevalence of infection to be 2.63% based on antigenemia and 1.03% based on microfilaremia (Dickson et al. 2018). No cases of lymphedema were found among participants, but 2.78% of males showed hydrocele. The present situation demonstrates promising features. Satisfactory measurements have been implemented with positive perspectives for eliminating the disease in the near future.
In Turkey, single case of filariasis was reported in an 11-year-old girl in a southern region, with swelling in both her legs (Cengiz et al. 2006). The species of the parasite has not been reported. Her family were reported to be free from the disease.
Japan is another country in Asia that has successfully eliminated LF (Ichimori et al. 2007). The initiative to combat the infection was started in the 1970s and ended in 1999. The high level of cooperation among the population was an important factor in eliminating not only LF but also many other parasitic diseases.
In Oman, the LF situation presents no serious concerns. Only sporadic cases have been reported, most of which were imported. A study of 250 children aged 17 to 18 years in 2004 detected no positive cases (Al Awaidy et al. 2010), and the authors concluded that LF is nonendemic in Oman. In another study from 1999 to 2013, 5 cases of filariasis were reported, of which 4 cases were travel-associated infections. The type of filariasis was not reported in this study (Al-Abri et al. 2015).
An important factor which has a decisive influence on the prognosis of LF is climate change. As a vector-borne infectious disease, LF is considered among the parasitic diseases affected by climate change. Accordingly, it is expected that LF could readily spread to new areas and worsen the situation (Short et al. 2017). Soil and plant canopy moisture levels are factors which directly influence the distribution of LF because they affect mosquito larvae breeding sites (Thompson et al. 1996). Changing temperature and precipitation patterns will thus affect soil moisture levels and mosquito populations. In Africa, it is reported that based on the level of climate change, the population at risk of LF may increase from 543 to 804 million to as much as 1.65–1.86 billion by 2050 (Slater and Michael 2012).
A limitation of our study was that some articles not accessible. Although we used some abstracts to obtain a clear-cut picture of the LF situation in Asia, the lack of access to information in some full texts may somewhat compromise the integrity of the output. To offset this drawback as far as possible, we tried to review all 48 countries individually to ensure reliability.
We found no cases of LF in other countries in Asia, although other kinds of filariasis were reported (Negahban et al. 2007; Parsa et al. 2020; Reddy 2013; Rokni 2008; Simón et al. 2012).
Conclusion
After the WHO announced a major initiative to eliminate LF in 2000, considerable progress has been made. Many countries have succeeded in eliminating the disease in accordance with the goals set by the WHO, while other countries should still take additional steps. Our study shows that in general, the outcomes have been satisfactory, and measures recommended by the WHO were observed to an adequate extent. One disappointing aspect is that unfortunately the region encompasses many political and social issues in some countries, including immigration, disruption, and poverty. These problems hinder efforts to attain all the aims proposed by the WHO to eliminate the disease in all countries.
According to evaluations conducted by the WHO and some governments in the region, LF is on track to be controlled and eliminated, yet some important factors including climate changes and especially the deadly new disease caused by SARS-CoV-2 will undoubtedly affect future efforts. Increasing declines in financial and economic resources may foreseeably prevent further efforts to control and eradicate filariasis. In order to continue the fight against the disease and prevent its spread, it is necessary for health authorities to consider the following points:
-
1.
Regular training for health workers
-
2.
Systematic surveillance management
-
3.
Direct Network Report system
-
4.
Establishing new and effective diagnostic methods
-
5.
TAS and morbidity datasets should be developed for post-elimination surveillance strategies
-
6.
Long-term reporting of new cases
-
7.
Patient access to care (lymphedema management and hydrocele surgery)
-
8.
Patient outreach and identification activities
-
9.
Integration of LF clinical care into the primary health care system
-
10.
Establishing a system of travel health service, e.g., increasing physicians’ awareness of travel-associated infections and passenger inspection programs for countries with high LF endemicity due to high numbers of immigrants
-
11.
Eventually, five public health strategies recommended by the WHO to monitor neglected tropical diseases should be implemented by all involved countries: expansion of preventive chemotherapy, intensified case detection and case management, improved vector control, appropriate veterinary public health measures, and provision of safe water, sanitation, and hygiene.
References
Abdulcader MHM, Rajakone P, Rajendran K, Aponso L (1966) Age, sex, and house distribution of Wuchereria bancrofti Microfilaremia in Ceylon. Am J Trop Med Hyg 15:519–522. https://doi.org/10.4269/ajtmh.1966.15.519
Addiss DG (2010) Global elimination of lymphatic filariasis: addressing the public health problem. PLoS Negl Trop Dis 4:e741. https://doi.org/10.1371/journal.pntd.0000741
Agrawal VK, Sashindran VK (2006) Lymphatic filariasis in India: problems, challenges and new initiatives. Med J Armed Forces India 62:359–362. https://doi.org/10.1016/s0377-1237(06)80109-7
Ahmad N (1945) Problems of filariasis with reference to post-war planning in India. J Indian Med Assoc 14:306–309
Al Awaidy ST, Bawikar S, Patel PK, Kurup P, Sonal GS, Al Mahrooqi S, Ramzy R (2010) Absence of lymphatic filariasis infection among secondary-school children in Oman. East Mediterr Health J 16:1059–1063
Al-Abd NM, Nor ZM, Ahmed A, Al-Adhroey AH, Mansor M, Kassim M (2014) Lymphatic filariasis in Peninsular Malaysia: a cross-sectional survey of the knowledge, attitudes, and practices of residents. Parasit Vectors 7:545. https://doi.org/10.1186/s13071-014-0545-z
Al-Abri SS, Abdel-Hady DM, Al Mahrooqi SS, Al-Kindi HS, Al-Jardani AK, Al-Abaidani IS (2015) Epidemiology of travel-associated infections in Oman 1999-2013: a retrospective analysis. Travel Med Infect Dis 13:388–393. https://doi.org/10.1016/j.tmaid.2015.08.006
Al-Shaham AA, Sood S (2010) Recurrent furunculosis as a cause of isolated penile lymphedema: a case report. J Med Case Rep 4:196. https://doi.org/10.1186/1752-1947-4-196
Anonymous (1991) Major achievements and experience in filariasis control in the People’s Republic of China. National Technical Steering Group for Filariasis Control and Research. Chin Med J (Engl) 104:446–453
Ashrafi K, Golchai J, Geranmayeh S (2010) Human subcutaneous dirofilariasis due to Dirofilaria (Nochtiella) repens: clinically suspected as cutaneous fascioliasis. Iran J Public Health 39:105–109
Aye NN, Lin Z, Lon KN, Linn NYY, Nwe TW, Mon KM, Ramaiah K, Betts H, Kelly-Hope LA (2018) Mapping and modelling the impact of mass drug adminstration on filariasis prevalence in Myanmar. Infect Dis Poverty 7:56. https://doi.org/10.1186/s40249-018-0420-9
Cane L (2020) A bright future: eliminating lymphatic filariasis in Vietnam. https://medium.com/@RTI_INTL_DEV/a-bright-future-eliminating-lymphatic-filariasis-in-vietnam-776bae245c8c. Accessed 11 June 2020
Cengiz N, Savaş L, Uslu Y, Anarat A (2006) Filariasis in a child from southern Turkey: a case report. Turk J Pediatr 48:152–154
Chandrasena NT, Premaratna R, Samarasekera DS, de Silva NR (2016) Surveillance for transmission of lymphatic filariasis in Colombo and Gampaha districts of Sri Lanka following mass drug administration. Trans R Soc Trop Med Hyg 110:620–622. https://doi.org/10.1093/trstmh/trw067
Cheun HI, Kong Y, Cho SH, Lee JS, Chai JY, Lee JS, Lee JK, Kim TS (2009) Successful control of lymphatic filariasis in the Republic of Korea. Korean J Parasitol 47:323–335. https://doi.org/10.3347/kjp.2009.47.4.323
Cheun HI et al (2017) Follow-up study of patients previously diagnosed with lymphatic filariasis in Korea. Osong Public Health Res Perspect 8:421–424. https://doi.org/10.24171/j.phrp.2017.8.6.10
Chu BK, Deming M, Biritwum NK, Bougma WR, Dorkenoo AM, el-Setouhy M, Fischer PU, Gass K, Gonzalez de Peña M, Mercado-Hernandez L, Kyelem D, Lammie PJ, Flueckiger RM, Mwingira UJ, Noordin R, Offei Owusu I, Ottesen EA, Pavluck A, Pilotte N, Rao RU, Samarasekera D, Schmaedick MA, Settinayake S, Simonsen PE, Supali T, Taleo F, Torres M, Weil GJ, Won KY (2013) Transmission assessment surveys (TAS) to define endpoints for lymphatic filariasis mass drug administration: a multicenter evaluation. PLoS Negl Trop Dis 7:e2584. https://doi.org/10.1371/journal.pntd.0002584
Collaborators GDaH (2017) Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390:1260–1344. https://doi.org/10.1016/s0140-6736(17)32130-x
de Meillon B, Grab B, Sebastian A (1967) Evaluation of Wuchereria bancrofti infection in Culex pipiens fatigans in Rangoon, Burma. Bull World Health Organ 36:91–100
De-Jian S, Xu-Li D, Ji-Hui D (2013) The history of the elimination of lymphatic filariasis in China. Infect Dis Poverty 2:30. https://doi.org/10.1186/2049-9957-2-30
Department of Health Services (DoHS) T (2020) Kathmandu Lymphatic filariasis elimination. https://www.mohp.gov.np/eng/program/communicable-disease/lymphatic-fliariasis#:~:text=Lymphatic%20Filariasis%20(LF)%20is%20a,%3C1%25%20to%2039%25. Accessed 7 June 2020
Dickson BFR, Graves PM, Aye NN, Nwe TW, Wai T, Win SS, Shwe M, Douglass J, Bradbury RS, McBride WJ (2018) The prevalence of lymphatic filariasis infection and disease following six rounds of mass drug administration in Mandalay Region, Myanmar. PLoS Negl Trop Dis 12:e0006944. https://doi.org/10.1371/journal.pntd.0006944
El-Moamly AA, El-Sweify MA, Hafez MA (2012) Using the AD12-ICT rapid-format test to detect Wuchereria bancrofti circulating antigens in comparison to Og4C3-ELISA and nucleopore membrane filtration and microscopy techniques. Parasitol Res 111:1379–1383. https://doi.org/10.1007/s00436-012-2870-5
Epidemiology and Disease Control Division Teku K, Nepal (2018) Lymphatic filariasis elimination program Annual report (2017/18). http://www.edcd.gov.np/resources/download/lymphatic-filariasisannual-report-201718. Accessed 4 June 2020
Fang Y, Zhang Y (2019) Lessons from lymphatic filariasis elimination and the challenges of post-elimination surveillance in China. Infect Dis Poverty 8:66. https://doi.org/10.1186/s40249-019-0578-9
Foo PK, Tarozzi A, Mahajan A, Yoong J, Krishnan L, Kopf D, Blackburn BG (2011) High prevalence of Wuchereria bancrofti infection as detected by immunochromatographic card testing in five districts of Orissa, India, previously considered to be non-endemic. Trans R Soc Trop Med Hyg 105:109–114. https://doi.org/10.1016/j.trstmh.2010.10.006
Galvez Tan JZ (2003) The elimination of lymphatic filariasis: a strategy for poverty alleviation and sustainable development - perspectives from the Philippines. Filaria J 2:12. https://doi.org/10.1186/1475-2883-2-12
George S, Joy TM, Kumar A, Panicker KN, George LS, Raj M, Leelamoni K, Nair P (2019) Prevalence of neglected tropical diseases (leishmaniasis and lymphatic filariasis) and malaria among a migrant labour settlement in Kerala, India. J Immigr Minor Health 21:563–569. https://doi.org/10.1007/s10903-018-0767-9
Ghasemi E, Shamsinia S, Taghipour A, Anvari D, Bahadory S, Shariatzadeh SA, Kordi B, Majidiani H, Borji H, Chaechi Nosrati M, Yousefi A, Shams M (2020) Filarial worms: a systematic review and meta-analysis of diversity in animals from Iran with emphasis on human cases. Parasitology 147(9):909–921. https://doi.org/10.1017/S003118202000058X
Ginandjar P, Saraswati LD, Suparyanto D, Sakundarno M, Supali T (2018) The prevalence of lymphatic filariasis in elementary school children living in endemic areas: a baseline survey prior to mass drug administration in Pekalongan District-Indonesia. Iran J Public Health 47:1484–1492
Gutierrez Y, Misselevich I, Fradis M, Podoshin L, Boss JH (1995) Dirofilaria repens infection in northern Israel. Am J Surg Pathol 19:1088–1091. https://doi.org/10.1097/00000478-199509000-00014
Hafiz I, Graves P, Haq R, Flora MS, Kelly-Hope LA (2015) Clinical case estimates of lymphatic filariasis in an endemic district of Bangladesh after a decade of mass drug administration. Trans R Soc Trop Med Hyg 109:700–709. https://doi.org/10.1093/trstmh/trv084
Haleem A, Al Juboury M, Al Husseini H (2002) Filariasis: a report of three cases. Ann Saudi Med 22:77–79. https://doi.org/10.5144/0256-4947.2002.77
Hotez PJ, Ehrenberg JP (2010) Escalating the global fight against neglected tropical diseases through interventions in the Asia Pacific region. Adv Parasitol 72:31–53. https://doi.org/10.1016/s0065-308x(10)72002-9
Ichimori K, Graves PM, Crump A (2007) Lymphatic filariasis elimination in the Pacific: PacELF replicating Japanese success. Trends Parasitol 23:36–40. https://doi.org/10.1016/j.pt.2006.11.005
Iddawela D, Ehambaram K, Wickramasinghe S (2015) Human ocular dirofilariasis due to Dirofilaria repens in Sri Lanka. Asian Pac J Trop Med 8:1022–1026. https://doi.org/10.1016/j.apjtm.2015.11.010
Irvine MA, Kazura JW, Hollingsworth TD (2018) Understanding heterogeneities in mosquito-bite exposure and infection distributions for the elimination of lymphatic filariasis. Proc Biol Sci 285(1871):20172253. https://doi.org/10.1098/rspb.2017.2253
Iyengar MO (1953) Filariasis in Thailand. Bull World Health Organ 9:731–766
Jamail M, Andrew K, Junaidi D, Krishnan AK, Faizal M, Rahmah N (2005) Field validation of sensitivity and specificity of rapid test for detection of Brugia malayi infection. Trop Med Int Health 10:99–104. https://doi.org/10.1111/j.1365-3156.2004.01334.x
Jamshidi A, Jamshidi M, Mobedi I, Khosroara M (2008) Periocular dirofilariasis in a young woman: a case report. Korean J Parasitol 46:265–267. https://doi.org/10.3347/kjp.2008.46.4.265
Kabatereine NB, Malecela M, Lado M, Zaramba S, Amiel O, Kolaczinski JH (2010) How to (or not to) integrate vertical programmes for the control of major neglected tropical diseases in sub-Saharan Africa. PLoS Negl Trop Dis 4:e755. https://doi.org/10.1371/journal.pntd.0000755
Karim MJ, Haq R, Mableson HE, Sultan Mahmood ASM, Rahman M, Chowdhury SM, Rahman A, Hafiz I, Betts H, Mackenzie C, Taylor MJ, Kelly-Hope LA (2019) Developing the first national database and map of lymphatic filariasis clinical cases in Bangladesh: another step closer to the elimination goals. PLoS Negl Trop Dis 13(7):e0007542. https://doi.org/10.1371/journal.pntd.000754213:e0007542
Khan AM, Dutta P, Sarmah CK, Baruah NK, Das S, Pathak AK, Sarmah P, Hussain ME, Mahanta J (2015) Prevalence of lymphatic filariasis in a tea garden worker population of Dibrugarh (Assam), India after six rounds of mass drug administration. J Vector Borne Dis 52:314–320
Khieu V, Or V, Tep C, Odermatt P, Tsuyuoka R, Char MC, Brady MA, Sidwell J, Yajima A, Huy R, Ramaiah KD, Muth S (2018) How elimination of lymphatic filariasis as a public health problem in the Kingdom of Cambodia was achieved. Infect Dis Poverty 7:15. https://doi.org/10.1186/s40249-018-0394-7
Kron M, Walker E, Hernandez L, Torres E, Libranda-Ramirez B (2000) Lymphatic filariasis in the Philippines. Parasitol Today 16:329–333. https://doi.org/10.1016/S0169-4758(00)01705-1
Leang R, Socheat D, Bin B, Bunkea T, Odermatt P (2004) Assessment of disease and infection of lymphatic filariasis in Northeastern Cambodia. Trop Med Int Health 9:1115–1120. https://doi.org/10.1111/j.1365-3156.2004.01311.x
Lee J, Ryu JS (2019) Current status of parasite infections in Indonesia: a literature review. Korean J Parasitol 57:329–339. https://doi.org/10.3347/kjp.2019.57.4.329
Leonardo L, Hernandez L, Magturo TC, Palasi W, Rubite JM, de Cadiz A, Moendeg K, Fornillos RJ, Tabios IK, Mistica M, Fontanilla IK (2020) Current status of neglected tropical diseases (NTDs) in the Philippines. Acta Trop 203:105284. https://doi.org/10.1016/j.actatropica.2019.105284
Li CY, Chang YL, Lee YC (2013) Human pulmonary dirofilariasis coexisting with intercostal neurilemmoma: a case report and literature review. J Formos Med Assoc 112:644–647. https://doi.org/10.1016/j.jfma.2012.07.016
Mallawarachchi CH, Nilmini Chandrasena TGA, Premaratna R, Mallawarachchi S, de Silva NR (2018) Human infection with sub-periodic Brugia spp. in Gampaha District, Sri Lanka: a threat to filariasis elimination status. Parasit Vectors 11:68. https://doi.org/10.1186/s13071-018-2649-3
Mandal NN, Bal MS, Das MK, Achary KG, Kar SK (2010) Lymphatic filariasis in children: age dependent prevalence in an area of India endemic for Wuchereria bancrofti infection. Trop Biomed 27:41–46
Meyrowitsch DW, Toan ND, Hao HT, Dan NT, Michael E (1998) A review of the present status of lymphatic filariasis in Vietnam. Acta Trop 70:335–347. https://doi.org/10.1016/S0001-706X(98)00037-0
Mukhopadhyay AK (2010) Lymphatic filariasis in Andhra Pradesh Paper Mill Colony, Rajahmundry, India after nine rounds of MDA programme. J Vector Borne Dis 47:55–57
Mukhopadhyay AK, Patnaik SK, Babu PS (2007) Status of lymphatic filariasis in parts of east Godavari district of Andhra Pradesh, India. J Vector Borne Dis 44:72–74
Negahban S, Daneshbod Y, Atefi S, Daneshbod K, Sadjjadi SM, Hosseini SV, Bedayat GR, Abidi H (2007) Dirofilaria repens diagnosed by the presence of microfilariae in fine needle aspirates: a case report. Acta Cytol 51:567–570. https://doi.org/10.1159/000325796
Nithikathkul C, Wannapinyosheep S, Saichua P, Nithikathkul M (2006) Filariasis: the disease will come to be the problem of Thailand. JSHMR 24:6
Noordin R, Mohd Zain SN, Yunus MH, Sahimin N (2017) Seroprevalence of lymphatic filariasis among migrant workers in Peninsular Malaysia. Trans R Soc Trop Med Hyg 111:370–372. https://doi.org/10.1093/trstmh/trx062
Omar MS (1996) A survey of bancroftian filariasis among South-East Asian expatriate workers in Saudi Arabia. Trop Med Int Health 1:155–160. https://doi.org/10.1111/j.1365-3156.1996.tb00021.x
Ottesen EA (2000) The global programme to eliminate lymphatic filariasis. Trop Med Int Health 5:591–594. https://doi.org/10.1046/j.1365-3156.2000.00620.x
Parsa R, Sedighi A, Sharifi I, Bamorovat M, Nasibi S (2020) Molecular characterization of ocular dirofilariasis: a case report of Dirofilaria immitis in south-eastern Iran. BMC Infect Dis 20:520. https://doi.org/10.1186/s12879-020-05182-5
Pradhan SP, Shrestha I, Palikhey N, Uprety RP (1998) Epidemiological study of lymphatic filariasis in Gokarna village development committee of Kathmandu valley during August and Septem. J Nepal Hlth Res Council 2:13–17
Rahmah N, Lim BH, Azian H, Ramelah TS, Rohana AR (2003) Short communication: use of a recombinant antigen-based ELISA to determine prevalence of brugian filariasis among Malaysian schoolchildren near Pasir Mas, Kelantan-Thailand border. Trop Med Int Health 8:158–163. https://doi.org/10.1046/j.1365-3156.2003.01004.x
Rajagopalan PK, Das PK, Subramanian S, Vanamail P, Ramaiah KD (1989) Bancroftian filariasis in Pondicherry, south India: 1. Pre-control epidemiological observations. Epidemiol Infect 103:685–692. https://doi.org/10.1017/s0950268800031083
Raju K, Jambulingam P, Sabesan S, Vanamail P (2010) Lymphatic filariasis in India: epidemiology and control measures. J Postgrad Med 56:232–238. https://doi.org/10.4103/0022-3859.68650
Rana Krishna J (2003) A brief study on the epidemiology of filariasis in Nepal. J Nepal Med Assoc 11:155–168. https://doi.org/10.31729/jnma.1561
Rao RU, Samarasekera SD, Nagodavithana KC, Dassanayaka TDM, Punchihewa MW, Ranasinghe USB, Weil GJ (2017) Reassessment of areas with persistent Lymphatic Filariasis nine years after cessation of mass drug administration in Sri Lanka. PLoS Negl Trop Dis 11:e0006066. https://doi.org/10.1371/journal.pntd.0006066
Rao RU, Samarasekera SD, Nagodavithana KC, Goss CW, Punchihewa MW, Dassanayaka TDM, Ranasinghe USB, Mendis D, Weil GJ (2018) Comprehensive assessment of a hotspot with persistent Bancroftian filariasis in coastal Sri Lanka. Am J Trop Med Hyg 99:735–742. https://doi.org/10.4269/ajtmh.18-0169
Reddy MV (2013) Human dirofilariasis: an emerging zoonosis. Trop Parasitol 3:2–3
Research UNWBWSPf, Training in Tropical D, Mapping WUJPfH, World Health Organization. Division of Tropical D (1998) Research on rapid geographical assessment of Bancroftian filariasis. World Health Organization, Geneva
Rojanapanus S, Toothong T, Boondej P, Thammapalo S, Khuanyoung N, Santabutr W, Prempree P, Gopinath D, Ramaiah KD (2019) How Thailand eliminated lymphatic filariasis as a public health problem. Infect Dis Poverty 8:38. https://doi.org/10.1186/s40249-019-0549-1
Rokni MB (2008) The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol 102:283–295. https://doi.org/10.1179/136485908x300805
Rubis P, Chang MS, Nagum AJ, Jau JL (1981) Parasitological and entomological studies on filariasis in seven villages, Serian District, Sarawak, East Malaysia. Southeast Asian J Trop Med Public Health 12:30–35
Rubite JM (2018) Initiatives on NTDs in the Philippines. In: the First ASEAN LF Forum, Manila, Philippines, 11-12 July, 2018
Sabesan S, Raju KH, Subramanian S, Srivastava PK, Jambulingam P (2013) Lymphatic filariasis transmission risk map of India, based on a geo-environmental risk model. Vector Borne Zoonotic Dis 13:657–665. https://doi.org/10.1089/vbz.2012.1238
Senoo T (1943) Detection of microfilaria malayi brug in Korea. Nippon Kiseichu Gakkai Kiji 15:36
Senoo T, Lincicome RD (1951) Malayan filariasis; incidence and distribution in Southern Korea. U S Armed Forces Med J 2:1483–1489
Ruel E. Serrano, Tmong Udui, Vu Lam Binh, Morel E (2020) Three more countries eliminate lymphatic filariasis. https://www.who.int/westernpacific/news/detail/08-10-2018-three-more-countries-eliminate-lymphatic-filariasis. Accessed 11 June 2020
Shamsuzzaman AK et al (2017) The significant scale up and success of Transmission Assessment Surveys ‘TAS’ for endgame surveillance of lymphatic filariasis in Bangladesh: one step closer to the elimination goal of 2020. PLoS Negl Trop Dis 11:e0005340. https://doi.org/10.1371/journal.pntd.0005340
Sherchand JB, Obsomer V, Thakur GD, Hommel M (2003) Mapping of lymphatic filariasis in Nepal. Filaria J 2:7. https://doi.org/10.1186/1475-2883-2-7
Short EE, Caminade C, Thomas BN (2017) Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect Dis (Auckl) 10:1178633617732296. https://doi.org/10.1177/1178633617732296
Simón F, Siles-Lucas M, Morchón R, González-Miguel J, Mellado I, Carretón E, Montoya-Alonso JA (2012) Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microbiol Rev 25:507–544. https://doi.org/10.1128/cmr.00012-12
Slater H, Michael E (2012) Predicting the current and future potential distributions of lymphatic filariasis in Africa using maximum entropy ecological niche modelling. PLoS One 7:e32202. https://doi.org/10.1371/journal.pone.0032202
Solgi R, Sadjjadi SM, Mohebali M, Djadid ND, Raz A, Zakeri S, Zarei Z (2017) Susceptibility of Anopheles stephensi (Diptera: Culicidae) to Dirofilaria immitis (Spirurida: Onchocercidae). Russ J Nematol 25(2):121–127. https://doi.org/10.24411/0869-6918-2017-00005
Srividya A, Michael E, Palaniyandi M, Pani SP, Das PK (2002) A geostatistical analysis of the geographic distribution of lymphatic filariasis prevalence in southern India. Am J Trop Med Hyg 67:480–489. https://doi.org/10.4269/ajtmh.2002.67.480
Sun DJ, Chen PL (1992) Filariasis surveillance at the post-control stage in China. Southeast Asian J Trop Med Public Health 23:369–376
Supali T et al (2002) High prevalence of Brugia timori infection in the highland of Alor Island, Indonesia. Am J Trop Med Hyg 66:560–565. https://doi.org/10.4269/ajtmh.2002.66.560
Tavakolizadeh S, Mobedi I (2009) Orbital dirofilariasis in Iran: a case report. Korean J Parasitol 47:397–399. https://doi.org/10.3347/kjp.2009.47.4.397
Thompson DF, Malone JB, Harb M, Faris R, Huh OK, Buck AA, Cline BL (1996) Bancroftian filariasis distribution and diurnal temperature differences in the southern Nile delta. Emerg Infect Dis 2:234–235. https://doi.org/10.3201/eid0203.960313
Toothong T, Tipayamongkholgul M, Suwannapong N, Suvannadabba S (2015) Evaluation of mass drug administration in the program to control imported lymphatic filariasis in Thailand. BMC Public Health 15:975. https://doi.org/10.1186/s12889-015-2325-x
Torgerson PR, de Silva NR, Fèvre EM, Kasuga F, Rokni MB, Zhou XN, Sripa B, Gargouri N, Willingham AL, Stein C (2014) The global burden of foodborne parasitic diseases: an update. Trends Parasitol 30:20–26. https://doi.org/10.1016/j.pt.2013.11.002
Triteeraprapab S, Karnjanopas K, Pruksakorn C, Sai-Ngam A, Yentakam S, Loymak S (2001) Lymphatic filariasis caused by Brugia malayi in an endemic area of Narathiwat Province, southern of Thailand. J Med Assoc Thailand = Chotmaihet Thangphaet 84(Suppl 1):S182–S188
Upadhyayula SM, Mutheneni SR, Kadiri MR, Kumaraswamy S, Nagalla B (2012) A cohort study of lymphatic filariasis on socio economic conditions in Andhra Pradesh, India. PLoS One 7:e33779. https://doi.org/10.1371/journal.pone.0033779
Urbani C (1997) Control of schistosomiasis and other helminthiasis in Cambodia. Me´decins Sans Frontie`res Switzerland, internal report n/d
Utzinger J, Bergquist R, Olveda R, Zhou XN (2010) Important helminth infections in Southeast Asia diversity, potential for control and prospects for elimination. Adv Parasitol 72:1–30. https://doi.org/10.1016/s0065-308x(10)72001-7
WHO (2004) Report on the mid-term assessment of microfilaraemia reduction in sentinel sites of 13 countries of the global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec 79:457–468. http://www.who.int/neglected_diseases/resources/who_wer7940/en/. Accessed 17 June 2020
WHO (2011) Global programme to eliminate lymphatic filariasis: a manual for National Elimination Programmes (Monitoring and Epidemiological Assessment of Mass Drug Administration). http://www.who.int/lymphatic_filariasis/resources/9789241501484/. Accessed 14 Jun2 2020
WHO (2013) Towards eliminating lymphatic filariasis: progress in the South-East Asia Region (2001–2011).
WHO (2014) Global programme to eliminate lymphatic filariasis: progress report, 2013. Wkly Epidemiol Rec 89:409–418
WHO (2016) Maldives and Sri Lanka eliminate lymphatic filariasis. http://www.searo.who.int/mediacentre/releases/2016/1626/en/. Accessed 3 June 2020
WHO (2020) Neglected tropical diseases. https://www.who.int/neglected_diseases/diseases/en/. Accessed 3 June 2020
Wibawa T, Satoto TBT (2016) Magnitude of neglected tropical diseases in Indonesia at postmillennium development goals era. J Trop Med 2016:5716785–5716789. https://doi.org/10.1155/2016/5716785
Yap LF, Ramachandran CP, Balasingam E (1968) A parasitological study of Pulau Pinang and Pulau Perhentian Kechil, off Trengganu, West Malaysia. I. Malaria and filariasis. Med J Malaya 23:118–122
Acknowledgments
The sincere cooperation of Dr A Alizadeh, is highly appreciated. We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for editing the English in the manuscript.
Availability of data and material
All data, analyses, and Excel spreadsheets are available on request.
Funding
The study was funded by a grant awarded by the National Institute for Medical Research Development, Ministry of Health and Medical Education, Iran (No. 977187).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Negar BIZHANI, Saeideh HASHEMI HAFSHEJANI1, and Mohammad Bager ROKNI. Analysis and graph preparation were performed by Neda MOHAMMADI and Mehdi Rezaei. The first draft of the manuscript was written by Mohammad Bager ROKNI and Negar BIZHANI. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Code availability
Not applicable
Additional information
Section Editor: Ramaswamy Kalyanasundaram
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bizhani, N., Hashemi Hafshejani, S., Mohammadi, N. et al. Lymphatic filariasis in Asia: a systematic review and meta-analysis. Parasitol Res 120, 411–422 (2021). https://doi.org/10.1007/s00436-020-06991-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06991-y