Abstract
Purpose
The gut microbiota is hypothesized as a prognostic biomarker for cancer immunotherapy. Antibiotic-induced dysbiosis negatively affects the clinical outcomes of immunotherapy. However, the effect of dysbiosis on the efficacy and safety of Chemoimmunotherapy (chemo-IOs), the frontline standard of care, in advanced non-small cell lung cancer (NSCLC) remains unknown. We aimed to compare the efficacy and safety of chemo-IOs in patients exposed to antibiotics before treatment with those of patients who were not exposed.
Methods
We retrospectively reviewed patients with advanced NSCLC treated with first-line chemo-IOs between 2018 and 2020 at the National Cancer Center Hospital. The patients were divided into two groups: those exposed to antibiotics within 30 days before induction therapy (ABx group) and those did not antibiotics (Non-ABx group). Propensity score matching was used to control for potential confounding factors. Clinical outcomes including progression-free survival (PFS), overall survival (OS), and immune-related adverse events (irAEs) were compared.
Results
Of 201 eligible patients, 21 were in the ABx group, and 42 were in the non-ABx group after propensity score matching. No differences in PFS or OS emerged between the two groups (ABx group vs. Non-ABx group) (PFS:7.0 months vs. 6.4 months, hazard ratio [HR] 0.89; 95% confidence interval [CI], 0.49–1.63, OS:20.4 months vs. 20.1 months, HR 0.87; 95% CI 0.44–1.71). The frequency of irAEs before propensity score matching was similar across any-grade irAEs (39.4% vs. 42.9%) or grade 3 or higher irAEs (9.1% vs. 11.3%).
Conclusion
Antibiotic-induced dysbiosis may not affect the efficacy of chemo-IOs in patients with advanced NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard of care for previously untreated advanced non-small cell lung cancer (NSCLC) is combined cancer immunotherapy (IO) and cytotoxic chemotherapy (chemo-IO) (Gandhi et al. 2018; Paz-Ares et al. 2018; Socinski et al. 2018; Mok et al. 2019; West et al. 2019; Nishio et al. 2021). Although programmed cell death ligand-1 (PD-L1) expression has been used as a biomarker to stratify prognosis in many trials, its usefulness in predicting IO response is limited. The CheckMate 227 study found that nivolumab plus ipilimumab was effective, regardless of PD-L1 expression levels (Hellmann et al. 2019). Thus, additional biomarkers are needed to predict the efficacy of IO and immune-related adverse events (irAEs) when selecting the most appropriate patients.
Gut microbiota plays a crucial role in maintaining host immune and metabolic homeostasis, and dysbiosis has been linked to various disorders (Nicholson et al. 2012). Studies have shown that specific gut microbiota species and diversity can affect IO efficacy and irAEs (Vétizou et al. 2015; Becattini et al. 2016; Korpela et al. 2016; Routy et al. 2018a; Soularue et al. 2018; Hakozaki et al. 2020). Therefore, the gut microbiota is a potential biomarker for predicting IO efficacy and safety. Antibiotics-induced dysbiosis has consistently been shown to have negative therapeutic effects on IO monotherapy in various types of cancer, including NSCLC (Wilson et al. 2020; Chalabi et al. 2020; Lurienne et al. 2020; Tsikala-Vafea et al. 2021; Cortellini et al. 2021a; Derosa et al. 2021). However, several retrospective studies mainly in Europe and North America have shown that antibiotic-induced dysbiosis does not negatively affect the therapeutic efficacy of chemo-IOs (Cortellini et al. 2021b; Hopkins et al. 2022). As the composition of the gut microbiota depends on geographic sites and dietary habits, it remains unclear whether antibiotic-induced dysbiosis influences the effectiveness and safety of chemo-IOs, particularly in Japan or other Asian countries (Derosa et al. 2021).
The purpose of this study was to investigate whether the use of antibiotics affects the efficacy and safety of chemo-IOs in the Japanese population and to determine whether dysbiosis prior to chemo-IOs can be used as a biomarker for predicting the efficacy and safety of Chemoimmunotherapy.
Patients and methods
Study design and patients
This retrospective cohort study enrolled consecutive patients with advanced NSCLC who underwent pembrolizumab or atezolizumab plus platinum-based chemotherapy between December 2018 and December 2020 at the National Cancer Center Hospital, Tokyo, Japan. Patients with oncogenic driver gene mutations after the failure of tyrosine kinase inhibitors were also included. Patients who had received cytotoxic chemotherapy previously or had participated in clinical trials were excluded. As antibiotics-induced dysbiosis within approximately 1 month of IOs administration was shown to impact treatment efficacy, we divided the patients into two groups: those treated with antibiotics within 30 days prior to induction therapy (ABx group) and those who did not undergo antibiotic treatment (Non-ABx group) (Wilson et al. 2020; Derosa et al. 2021).
Data collection
Baseline patient characteristics prior to induction therapy, including PD-L1 status, and clinical outcomes, were collected from the electronic medical records (EMRs). The following information was also extracted: antibiotic type, route of administration, duration, and purpose of antibiotic treatment; concomitant medications such as proton pump inhibitors (PPIs), antihistamine blockers (H2B), and probiotics within 30 days of starting induction therapy; and factors related to antibiotic usages such as C-reactive protein (CRP) levels. Baseline maximum tumor size of the lung at the initiation of chemo-IOs was also investigated. PD-L1 expression levels were determined using a PD-L1 IHC 22C3 pharmDx (Agilent Technologies, Santa Clara, CA, USA). Tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1 (Eisenhauer et al. 2009).
The attending physicians collected data on irAEs which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 (National Cancer Institute Cancer Therapy Evaluation Program (2017)). All irAEs observed from the date of starting Chemo-IOs to the cutoff date were collected from the EMRs. Data collection was conducted until the cutoff date of October 31, 2022. Structured data (predefined, formatted, and coded data) were extracted directly from the EMRs. Unstructured data (from written case notes) or data not directly extractable were collected by KT. All extracted data were downloaded for this study. Data collection depended on data availability, quality, and validity, assessed through data due diligence to ensure data conformity, completeness, and plausibility.
Evaluation
The overall response rate (ORR) was defined as the percentage of patients with the best overall response either as a complete or partial response. Survival curves of progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method. PFS was defined as the time from the start of induction therapy to disease progression, death due to any cause. Patients without progression were censored at the last follow-up. OS was defined as the time from the start of induction therapy to death due to any cause. Patients without death were censored at the last follow-up.
Statistical analysis
Continuous variables such as age, maximum tumor size, and CRP level were dichotomized according to their median values.
The primary interest of this study is to examine whether an antibiotic-induced dysbiosis may be associated with patient outcomes. Considering potential confounding variables regarding ABx and non-ABx groups, a propensity score was estimated using a multivariable logistic regression model including histology, stage, smoking status, maximum tumor size, use of PPIs/H2B, and elevated CRP levels as covariates. Continuous variables including the maximum tumor size and CRP levels were dichotomized, and categorized by the median. The nearest neighbor 1:2 matching was employed with a caliper width of 0.2 standard deviations of the logit of the estimated propensity score.
For propensity score matched cohort, we performed univariable logistic regression for ORR and univariable Cox regression analysis for PFS and OS to estimate odds ratio and hazard ratios (HRs) with their 95% confidence intervals (CIs). As a benchmark, using propensity score unmatched cohort, their corresponding multivariable analyses were performed including the same covariates as used for estimating the propensity score.
All statistical analyses were conducted using EZR (Easy R) statistical software version 1.54 (Kanda 2013). All reported p-values were two-sided. This study was approved by the Committee of the National Cancer Center Hospital (2015–355). The opportunity to refuse this study by subjects was guaranteed with details of this study available to the public.
Results
Patient characteristics
Of the 202 patients who underwent first-line chemo-IO for advanced NSCLC, 201 were included in this study (Fig. 1). Prior to propensity score matching, 33 patients (15.8%) were in the ABx group and 168 (80.8%) were in the non-ABx group. After propensity score matching (PSM), 21 and 42 patients were included in the ABx and non-ABx groups, respectively.
Baseline patient characteristics are summarized in Table 1. In the overall cohort prior to PSM, the median age was 66.0 years (interquartile range [IQR] 58.0–71.0), 67.7% of patients were male, 93.0% had PS 0–1, and 78.1% were smokers. Eighty (39.8%) patients used PPIs/H2B and 5 (2.5%) patients used probiotics. Compared to the non-ABx group, the ABx group had more patients with squamous cell carcinoma ([ABx group vs. Non-ABx group] 42.4% vs. 13.7%), tumor size of 40 mm or larger (72.7% vs. 38.1%), and elevated CRP (≥ 1.04 mg/dl) (75.8% vs. 45.2%). Baseline variables used for estimating propensity score were tended to be well balanced between the two groups.
In the ABx group, prophylaxis was the most common reason for using antibiotics ([before PSM] 54.5%, [after PSM] 52.6%), particularly for pneumonia during bronchoscopy ([before PSM] 42.4%, [after PSM] 42.9%) (Table 2). Pneumonia was the most common cause for curative antibiotic therapy ([before PSM] 33.3%, [after PSM] 42.9%). Most patients were prescribed anaerobic-spectrum agents ([before PSM] 78.8%, [after PSM] 85.7%), including penicillin and beta-lactamase inhibitor combinations ([before PSM] 75.8%, [after PSM] 84.2%).
Efficacy following propensity score matching
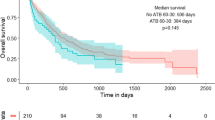
The ABx group had a median follow-up period of 45.9 months, while the non-ABx group had a median follow-up period of 47.2 months. The ORR showed no difference between the two groups (52.1% [95% CI 29.8–74.3] vs. 45.2% [95% CI 29.8–61.3%]) (Supplementary Table S1). The median PFS and OS were also not different between the two groups (PFS:7.0 months vs. 6.4 months, HR 0.89 [95% CI:0.49–1.63]; OS:20.4 months vs. 20.1 months, HR 0.87 [95% CI:0.44–1.71]) (Fig. 2a, b). Subgroup analyses for PFS and OS showed no differences among the baseline patient characteristics (Fig. 3a, b). Subgroup analysis of the ABx group based on the administration route (intravenous vs. oral), duration (≥ 1 week vs. < 1 week), spectrum (anaerobic vs. non-anaerobic), and purpose (curative vs. prophylaxis) of antibiotics did not reveal any differences in PFS or OS between the two groups (Supplementary Fig. S1).
Survival efficacy following propensity score matching. Kaplan–Meier estimates of progression-free survival (PFS) (A) and overall survival (OS) (B) between patients treated with antibiotics 30 days prior to induction therapy (ABx group) and those who did not receive antibiotics (Non-ABx group) after propensity score matching. CI, confidence interval; HR, hazard ratio
Subgroup analysis for survival efficacy following propensity score matching. Forest plots showing PFS (A) and OS (B) between patients treated with antibiotics 30 days before induction therapy (ABx group) and those who did not receive antibiotics (Non-ABx group) according to subgroup. aOncogenic driver mutations include EGFR, ALK, ROS-1, BRAF, MET exon 14 skipping, RET, and NTRK. CI, confidence interval; CRP, C-reactive protein; NE, not evaluated; Non-SQC, non-squamous cell carcinoma; PD-L1, programmed cell death-ligand 1; PPI/H2B, proton pump inhibitors/antihistamine blockers; PS, Eastern Cooperative Oncology Group performance status; SQC, squamous cell carcinoma; Tmax, maximum tumor size at the start of induction therapy
Multivariable analysis of efficacy prior to propensity score matching
We conducted a multivariable analysis of the cohort before PSM for ORR, PFS, and OS (Supplementary Table S2). There was no difference between the ABx group and the non-ABx group (ORR: Odds ratio 0.69 [95% CI 0.28–1.71], PFS: HR 0.91 [95% CI 0.56–1.49], OS: HR 0.99 [95% CI 0.57–1.73]). Elevated CRP (≥ 1.04 mg/dl) was a factor for worse PFS and OS (PFS: HR 2.30 [95% CI 1.49–3.55], OS: HR 3.00 [95% CI 1.80–5.01]).
Immune-related adverse events before propensity score matching
There was no difference in the frequency of irAEs between the ABx and non-ABx groups (39.4% vs. 42.9%) (Table 3). There was also no difference in the frequency of grade 3 or higher irAEs between the two groups (9.1% vs. 11.3%). Although the frequencies of interstitial lung disease, colitis, and skin reactions were higher in the non-ABx group compared to the ABx group, the frequency of grade 3 or higher serious adverse events did not differ between the two groups.
Discussion
Exposure to antibiotics within 30 days prior to the administration of chemo-IOs did not affect efficacy or safety. Although the group exposed to antibiotics may represent a population with a poor prognosis, similar results were obtained after adjusting for background factors. The reproducibility of the results was confirmed by the duration and route of administration of antibiotics, as well as anaerobic-spectrum antibiotics.
Antibiotics not only kill pathogens but also disrupt the diversity and composition of the gut microbiota, thereby altering the immune environment (Morgun et al. 2015). To succeed in cancer IO, it is important to accelerate the cancer immune cycle (Chen and Mellman 2013). Although the mechanism by which the gut microbiota affects the tumor immune environment is complex and not yet fully understood, tumor antigenicity and adjuvanticity are associated with antitumor immune responses. Antigenicity allows antigen mimicry between bacterial and tumor antigens to prime antitumor T cells (Zitvogel et al. 2016; Derosa et al. 2021). Adjuvanticity shows that pathogen recognition receptors, also called pattern recognition receptors, are activated by the microbiome and stimulate cytokines and interferons, resulting in the modulation of the immune tonus (Zitvogel et al. 2016; Derosa et al. 2021). These mechanisms facilitate the cancer immunity cycle. However, not all bacteria in the gut microbiome are associated with antitumor immune responses. Specific bacterial species, including Akkermansia muciniphila, Bacteroides fragilis, Bifidobacterium spp., Collinsella spp., and Ruminococcas spp., and their diversity and differential abundance are beneficial to the tumor immune environment (Vétizou et al. 2015; Matson et al. 2018; Routy et al. 2018b; Hakozaki et al. 2020). Antibiotics eliminate these beneficial bacteria and reduce their diversity, which has negative clinical effects on IOs. Indeed, the use of antibiotics before anti-PD-1/PD-L1 or anti-CTLA4 antibody monotherapy was associated with negative clinical outcomes in several meta-analyses and prospective studies, regardless of cancer type and geographic region (Lurienne et al. 2020; Wilson et al. 2020; Derosa et al. 2021; Matson et al. 2018).
Cytotoxic chemotherapy induces immunogenic cell death via neoantigens released from direct damage to cancer cells, resulting in enhanced efficacy when combined with IO successfully (Galluzzi et al. 2015). The gut microbiota stimulates immune cells from the bone marrow to produce reactive oxygen species (Iida et al. 2013). As a result, oxidative stress in tumors enhances DNA damage by platinum-based agents (Iida et al. 2013). Mice treated with antibiotics show reduced antitumor responses (Iida et al. 2013). Although these results suggest that antibiotic exposure before chemo-IOs may negatively impact clinical outcomes, our results are contradictory. Cytotoxic chemotherapy disrupts the gut mucosal barrier and alters the gut microbiota (Viaud et al. 2013). Although the underlying mechanism remains unclear, chemotherapy may overcome its negative effects. The addition of chemotherapy to IO may counteract the negative effects of antibiotic exposure. In the clinical setting, a European multicenter retrospective study supported our results that antibiotic exposure before chemo-IOs had no effect on efficacy (Cortellini et al. 2021b). Our results are the first to suggest that the efficacy of chemo-IOs does not depend on the gut microbiota in the Japanese population.
The gut microbiota is responsible for the occurrence of irAEs, which are characterized as IO toxicities. Although the mechanism remains unknown, activated T cells react mutually with tumors and healthy organ antigens and may be associated with increased levels of pre-existing autoantibodies, increased levels of inflammatory cytokines, and enhanced complement-mediated inflammation due to the direct connection of cytotoxic antibodies (Postow et al. 2018). The impact of gut microbiota on tumor immunity is similar to that of irAEs in terms of antigenicity. It is noteworthy that the administration of antibiotics before IOs has been shown to disrupt the gut microbiota and subsequently reduce the incidence of irAEs (Kostine et al. 2021; Neo et al. 2022). Certain bacterial species, such as Bacteroides spp., have been found to promote anti-tumor immune responses and confer resistance to immune-related colitis (Dubin et al. 2016). Although the use of cytotoxic chemotherapy enhances the effectiveness of IOs, the administration of antibiotics before chemotherapy may increase the risk of irAEs. Although the underlying mechanism is unclear, genetic factors such as HLA-A have been linked to irAEs, and various other factors may also contribute to their occurrence (Dubin et al. 2016). However, our results do not allow us to draw any conclusions regarding the safety impact of antibiotic exposure prior to chemo-IOs administration.
This study has several limitations. First, the retrospective, single-center design of this study restricts the generalizability of the results to other populations. Although the intestinal environment is significantly influenced by environmental and dietary habits, these factors cannot be determined from medical records. Additionally, patients who grew up abroad were underrepresented in our Japanese cancer center; thus, these findings should be generalized to other populations with caution. Second, the sample size of patients treated with probiotics was too small to draw conclusions regarding their efficacy in restoring antitumor responses (Tomita et al. 2020). Third, although propensity score matching and multivariable analysis were used to adjust for confounding factors, the use of antibiotics may have been associated with a poorer prognosis in certain populations. However, due to the small sample size, these strategies cannot adjust for all potential confounding factors in order to obtain reliable estimates of the hazard ratios for the PFS and OS. Fourth, the fecal microbiota was not identified using metagenomic analysis, which could have further clarified the role of the gut microbiota in this context. Further studies using metagenomic analyses are currently underway in Japan (Shiraishi et al. 2022). Finally, the CheckMate 9LA regimen, a combination of immunotherapy and cytotoxic chemotherapy, was excluded from the analysis because of its unavailability at the time of the study (John et al. 2022).
Conclusion
In conclusion, our study indicates that the administration of antibiotics within 30 days before chemo-IOs does not seem to significantly affect the effectiveness of treatment in the Japanese population.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABx:
-
Antibiotics
- chemo-IO:
-
Combined cytotoxic chemotherapy and cancer immunotherapy
- Cis:
-
Confidence intervals
- CRP:
-
C-reactive protein
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- EMRs:
-
Electronic medical records
- HRs:
-
Hazard ratios
- H2B:
-
Anti-histamine blockers
- IO:
-
Cancer immunotherapy
- IQR:
-
Interquartile range
- irAEs:
-
Immune-related adverse events
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PD-L1:
-
Programmed cell death ligand-1
- PFS:
-
Progression-free survival
- PPI:
-
Proton pump inhibitors
- PSM:
-
Propensity score matching
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
References
Becattini S, Taur Y, Pamer EG (2016) Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med 22:458–478. https://doi.org/10.1016/j.molmed.2016.04.003
Chalabi M, Cardona A, Nagarkar DR et al (2020) Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol 31:525–531. https://doi.org/10.1016/j.annonc.2020.01.006
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10. https://doi.org/10.1016/j.immuni.2013.07.012
Cortellini A, Di Maio M, Nigro O et al (2021a) Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J Immunother Cancer 9:e002421. https://doi.org/10.1136/jitc-2021-002421
Cortellini A, Ricciuti B, Facchinetti F et al (2021b) Antibiotic-exposed patients with non-small-cell lung cancer preserve efficacy outcomes following first-line chemo-immunotherapy. Ann Oncol 32:1391–1399. https://doi.org/10.1016/j.annonc.2021.08.1744
Derosa L, Routy B, Desilets A et al (2021) Microbiota-centered interventions: The next breakthrough in immuno-oncology? Cancer Discov 11:2396–2412. https://doi.org/10.1158/2159-8290.CD-21-0236
Dubin K, Callahan MK, Ren B et al (2016) Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. https://doi.org/10.1038/ncomms10391
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Galluzzi L, Buqué A, Kepp O et al (2015) Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28:690–714
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/nejmoa1801005
Hakozaki T, Richard C, Elkrief A et al (2020) The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res 8:1243–1250. https://doi.org/10.1158/2326-6066.CIR-20-0196
Hellmann MD, Paz-Ares L, Bernabe Caro R et al (2019) Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381:2020–2031. https://doi.org/10.1056/nejmoa1910231
Hopkins AM, Badaoui S, Kichenadasse G et al (2022) Efficacy of atezolizumab in patients with advanced NSCLC receiving concomitant antibiotic or proton pump inhibitor treatment: pooled analysis of five randomized control trials. J Thorac Oncol 17:758–767. https://doi.org/10.1016/j.jtho.2022.02.003
Iida N, Dzutsev A, Stewart CA et al (2013) Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science (80–) 342:967–970. https://doi.org/10.1126/science.1240527
John T, Sakai H, Ikeda S et al (2022) First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in advanced non-small cell lung cancer: a subanalysis of Asian patients in CheckMate 9LA. Int J Clin Oncol 27:695–706. https://doi.org/10.1007/s10147-022-02120-0
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Korpela K, Salonen A, Virta LJ et al (2016) Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun 7:10410. https://doi.org/10.1038/ncomms10410
Kostine M, Mauric E, Tison A et al (2021) Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur J Cancer 157:474–484. https://doi.org/10.1016/j.ejca.2021.08.036
Lurienne L, Cervesi J, Duhalde L et al (2020) NSCLC immunotherapy efficacy and antibiotic use: a systematic review and meta-analysis. J Thorac Oncol 15:1147–1159. https://doi.org/10.1016/j.jtho.2020.03.002
Matson V, Fessler J, Bao R et al (2018) The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science (80–) 359:104–108. https://doi.org/10.1126/science.aao3290
Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393:1819–1830. https://doi.org/10.1016/S0140-6736(18)32409-7
Morgun A, Dzutsev A, Dong X et al (2015) Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut 64:1732–1743. https://doi.org/10.1136/gutjnl-2014-308820
National Cancer Institute Cancer Therapy Evaluation Program (2017) Common terminology criteria for adverse events (CTCAE) | Protocol Development | CTEP. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 1 Apr 2023
Neo SY, Jing X, Tong L et al (2022) Tumor MHC class I expression alters cancer-associated myelopoiesis driven by host NK cells. J Immunother Cancer 10:1–7. https://doi.org/10.1136/jitc-2022-005308
Nicholson JK, Holmes E, Kinross J et al (2012) Host-gut microbiota metabolic interactions. Science (80–) 108:1262–1268. https://doi.org/10.1126/science.1223813
Nishio M, Barlesi F, West H et al (2021) Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol 16:653–664. https://doi.org/10.1016/j.jtho.2020.11.025
Paz-Ares L, Luft A, Vicente D et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051. https://doi.org/10.1056/nejmoa1810865
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168. https://doi.org/10.1056/nejmra1703481
Routy B, Gopalakrishnan V, Daillère R et al (2018a) The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol 15:382–396. https://doi.org/10.1038/s41571-018-0006-2
Routy B, Le Chatelier E, Derosa L et al (2018b) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (80–) 359:91–97. https://doi.org/10.1126/science.aan3706
Shiraishi Y, Hakozaki T, Nomura S et al (2022) A multicenter, randomized a multicenter, randomized phase III study comparing platinum combination chemotherapy plus pembrolizumab with platinum combination chemotherapy plus nivolumab and ipilimumab for treatment-naive advanced non-small cell lung cancer. Clin Lung Cancer 23:e285–e288. https://doi.org/10.1016/j.cllc.2021.10.012
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288–2301. https://doi.org/10.1056/nejmoa1716948
Soularue E, Lepage P, Colombel JF et al (2018) Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 67:2056–2067. https://doi.org/10.1136/gutjnl-2018-316948
Tomita Y, Ikeda T, Sakata S et al (2020) Association of probiotic clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res 8:1236–1242. https://doi.org/10.1158/2326-6066.CIR-20-0051
Tsikala-Vafea M, Belani N, Vieira K et al (2021) Use of antibiotics is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Int J Infect Dis 106:142–154. https://doi.org/10.1016/j.ijid.2021.03.063
Vétizou M, Pitt JM, Daillère R et al (2015) Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (80–) 350:1079–1084. https://doi.org/10.1126/science.aad1329
Viaud S, Saccheri F, Mignot G et al (2013) The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science (80–) 342:971–976. https://doi.org/10.1126/science.1240537
West H, McCleod M, Hussein M et al (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 tria. Lancet Oncol 20:924–937. https://doi.org/10.1016/S1470-2045(19)30167-6
Wilson BE, Routy B, Nagrial A, Chin VT (2020) The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies. Cancer Immunol Immunother 69:343–354. https://doi.org/10.1007/s00262-019-02453-2
Zitvogel L, Ayyoub M, Routy B, Kroemer G (2016) Microbiome and anticancer immunosurveillance. Cell 165:276–287
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study did not receive any specific grants from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
KT: conceptualization, methodology, investigation, formal analysis, writing—original draft preparation. YO: conceptualization, methodology, writing, review, editing, and supervision. SN: methodology, writing, review, and editing. AF, KM, YS, TY, YG, HH, NY, YO: conceptualization, methodology, writing, review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Tamura and Fukuda have nothing to disclose. Dr. Okuma reports grants from Roche, grants from ABBVIE K.K, personal fees from AstraZeneca, personal fees from EIi-Lilly K.K, personal fees from Bristol Meiers Squibb, personal fees from Pfizer Taiho Pharma Co. Ltd., personal fees from AstraZeneca Nippon Boehringer Ingelheim, personal fees from Chugai Pharma Co. Ltd., personal fees from Ono Pharma Co. Ltd., personal fees from Taiho Pharma Co. Ltd., outside the submitted work. Dr. Nomura reports grants and personal fees from AstraZeneca, personal fees from Chugai, grants from Amgen, personal fees from KyowaHakko, personal fees from JMDC, grants from Byer, personal fees from Asahi-Kasei Pharma, outside the submitted work. Dr. Masuda reports personal fees from ONO, personal fees from AstraZeneca, personal fees from Chugai, personal fees from Bristol Myers Squibb, outside the submitted work. Dr. Matsumoto reports grants from National Cancer Center Research and Development Fund, grants from Grant-in-Aid for Scientific Research on Innovative Areas, grants from Hitachi, Ltd. Grants, personal fees from Olympus, personal fees from AstraZeneca, personal fees from Novartis, personal fees from COOK, personal fees from AMCO INC, personal fees from Thermo Fisher Scientific, personal fees from Erbe Elektromedizin GmbH, personal fees from Fujifilm, personal fees from Chugai, personal fees from Eli Lilly, outside the submitted work. Dr. Shinno reports personal fees from BMS, personal fees from Chugai, personal fees from Astra Zeneca, personal fees from Eli Lilly, grants and personal fees from Ono, grants from Janssen, grants from Japan Clinical Research Operations K.K., outside the submitted work. Dr. Yoshida reports grants and personal fees from AMGEN, grants and personal fees from Astrazeneca, grants from Takeda, grants from Daiichi Sankyo, grants and personal fees from Ono, grants and personal fees from MSD, grants from Abbvie, grants and personal fees from Novartis, grants and personal fees from Chugai, grants and personal fees from BMS, personal fees from TAIHO, personal fees from Lilly, personal fees from Roche, personal fees from ArcherDX, outside the submitted work. Dr. Goto reports grants from AZK, grants and personal fees from Pfizer, grants from Abbvie, grants and personal fees from Eli Lilly, grants and personal fees from Bristol Myers Squibb, grants and personal fees from Ono, grants and personal fees from Novartis, grants from Kyorin, grants and personal fees from DaiichiSankyo, grants from Prefered Network, personal fees from Chugai, personal fees from Taiho, personal fees from Boehringer Ingelheim, personal fees from MSD, personal fees from Merck, personal fees from Thermo Fischer, personal fees from AstraZeneca, personal fees from Chugai, personal fees from Guardant Health Inc., personal fees from Illumina, outside the submitted work. Dr. Horinouchi reports grants and personal fees from MSD, grants from Abbvie, grants and personal fees from AstraZeneca, grants and personal fees from BMS, grants and personal fees from Ono, grants from Merck Biophama, grants from Daiichi-Sankyo, grants from Janssen, grants from Genomic Helath, grants and personal fees from Chugai, grants and personal fees from Roche, grants and personal fees from Novartis, personal fees from Eli Lilly, personal fees from Kyowa-Kirin, outside the submitted work. Dr. Yamamoto reports grants and personal fees from Chugai, grants from Taiho, grants and personal fees from Eisai, grants and personal fees from Lilly, grants from Quintiles, grants from Astellas, grants and personal fees from BMS, grants from Novartis, grants from Daiichi-Sankyo, grants and personal fees from Pfizer, grants and personal fees from Boehringer Ingelheim, grants from Kyowa-Hakko Kirin, grants from Bayer, grants and personal fees from ONO PHARMACEUTICAL CO., LTD, grants and personal fees from Takeda, grants from Janssen Pharma, grants from MSD, grants from Merck, personal fees from Sysmex, grants from GSK, grants from Sumitomo Dainippon, grants from Chiome Bioscience Inc., grants and personal fees from Otsuka, grants from Carna Biosciences, grants from Genmab, grants from Shionogi, personal fees from AstraZeneca, personal fees from Cimic, outside the submitted work. Dr. Ohe reports grants and personal fees from AstraZeneca, grants and personal fees from Chugai, grants and personal fees from Eli Lilly, grants and personal fees from ONO, grants and personal fees from BMS, grants and personal fees from Kyorin, grants from Dainippon-Sumitomo, grants and personal fees from Pfizer, grants and personal fees from Taiho, grants from Novartis, grants from Takeda, grants from Kissei, grants from Daiichi-Sankyo, grants from Janssen, grants from LOXO, personal fees from Boehringer Ingelheim, personal fees from Bayer, personal fees from MSD, personal fees from Nippon Kayaku, personal fees from Kyowa Hakko Kirin, personal fees from Celltrion, personal fees from Amgen, personal fees from AnHeeart Tharapeutics Inc., outside the submitted work.
Consent to participate
The opportunity to refuse this study by subjects was guaranteed with details of this study available to the public.
Ethics approval
This study was approved by the Committee of the National Cancer Center Hospital (2015–355).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tamura, K., Okuma, Y., Nomura, S. et al. Efficacy and safety of chemoimmunotherapy in advanced non-small cell lung cancer patients with antibiotics-induced dysbiosis: a propensity-matched real-world analysis. J Cancer Res Clin Oncol 150, 216 (2024). https://doi.org/10.1007/s00432-024-05649-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05649-x