Abstract
Purpose
Some metastatic colorectal cancer (mCRC) patients receive conversion surgery (CS), including metastasectomy after palliative chemotherapy. Although targeted agents significantly improved the outcomes, the clinical outcome of CS in the targeted agent era has not yet been thoroughly investigated.
Methods
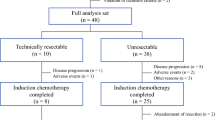
We analyzed the clinical data of 96 mCRC patients who initially had unresectable liver- and/or lung-limited metastases and underwent first-line cetuximab or bevacizumab plus FOLFIRI between January 2013 and June 2017.
Results
Liver-limited metastasis was seen in 44 patients (45.8%), lung-limited metastases in 21 patients (21.9%), and both liver and lung metastases in 31 patients (32.3%). Among them, 37 patients (38.5%) received cetuximab, and 59 patients (61.5%) received bevacizumab plus FOLFIRI. Overall response rate was 63.9% and 40.7%, respectively (p = 0.035). After median 8.7 (range 2.5–27.3) months, CS was performed in 11 patients (29.7%) in cetuximab group and 15 patients (25.4%) in bevacizumab group (p = 0.646). Median overall survival has not been reached in R0-resected patients (n = 23), during the median follow-up period of 22.5 (range 9.8–54.5) months. Median disease-free survival was 7.1 (95% CI 2.5–11.7) months: 11.0 (95% CI 3.1–19.0) months in cetuximab group and 3.2 (95% CI 0.0–7.8) months in bevacizumab group (p = 0.422). There was no progression after 18.5 months and disease-free survival reached a plateau at 19.9%.
Conclusions
A substantial proportion of patients could receive CS after cetuximab or bevacizumab plus FOLFIRI chemotherapy. R0-resected patients had excellent overall survival, although 80.1% of them eventually experienced recurrence. Some patients could achieve durable disease-free state.



Similar content being viewed by others
References
Bertotti A, Papp E, Jones S, Adleff V, Anagnostou V, Lupo B, Sausen M, Phallen J, Hruban CA, Tokheim C, Niknafs N, Nesselbush M, Lytle K, Sassi F, Cottino F, Migliardi G, Zanella ER, Ribero D, Russolillo N, Mellano A, Muratore A, Paraluppi G, Salizzoni M, Marsoni S, Kragh M, Lantto J, Cassingena A, Li QK, Karchin R, Scharpf R, Sartore-Bianchi A, Siena S, Diaz LA Jr, Trusolino L, Velculescu VE (2015) The genomic landscape of response to EGFR blockade in colorectal cancer. Nature 526(7572):263–267. https://doi.org/10.1038/nature14969
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. https://doi.org/10.3322/caac.21492
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, Konopke R, Stroszczynski C, Liersch T, Ockert D, Herrmann T, Goekkurt E, Parisi F, Kohne CH (2010) Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 11(1):38–47. https://doi.org/10.1016/S1470-2045(09)70330-4
Folprecht G, Gruenberger T, Bechstein W, Raab HR, Weitz J, Lordick F, Hartmann JT, Stoehlmacher-Williams J, Lang H, Trarbach T, Liersch T, Ockert D, Jaeger D, Steger U, Suedhoff T, Rentsch A, Kohne CH (2014) Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol 25(5):1018–1025. https://doi.org/10.1093/annonc/mdu088
François Quenet DE, Roca L, Goere D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, Marchal F, Loi V, Meeus P, De Forges H, Stanbury T, Paineau J, Glehen O (2018) A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol 36(suppl; abstr LBA):3503
Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q et al (2019) Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol 29(7):3564–3573
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Muller S, Link H, Niederle N, Rost A, Hoffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15(10):1065–1075. https://doi.org/10.1016/S1470-2045(14)70330-4
Holch JW, Demmer M, Lamersdorf C, Michl M, Schulz C, von Einem JC, Modest DP, Heinemann V (2017) Pattern and dynamics of distant metastases in metastatic colorectal cancer. Visc Med 33(1):70–75. https://doi.org/10.1159/000454687
Ji JH, Park SH, Lee J, Kim TW, Hong YS, Kim KP, Kim SY, Baek JY, Kang HJ, Shin SJ, Shim BY, Park YS (2013) Prospective phase II study of neoadjuvant FOLFOX6 plus cetuximab in patients with colorectal cancer and unresectable liver-only metastasis. Cancer Chemother Pharmacol 72(1):223–230. https://doi.org/10.1007/s00280-013-2190-1
Jung KW, Won YJ, Kong HJ, Lee ES, Community of Population-Based Regional Cancer R (2018) Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat 50(2):303–316. https://doi.org/10.4143/crt.2018.143
Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G (2012) Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 4:283–301. https://doi.org/10.2147/CLEP.S34285
Lv W, Zhang GQ, Jiao A, Zhao BC, Shi Y, Chen BM, Zhang JL (2017) Chemotherapy plus cetuximab versus chemotherapy alone for patients with KRAS wild type unresectable liver-confined metastases colorectal cancer: an updated meta-analysis of RCTs. Gastroenterol Res Pract 2017:8464905. https://doi.org/10.1155/2017/8464905
Meyerhardt JA, Mayer RJ (2005) Systemic therapy for colorectal cancer. N Engl J Med 352(5):476–487. https://doi.org/10.1056/NEJMra040958
Modest DP, Denecke T, Pratschke J, Ricard I, Lang H, Bemelmans M, Becker T, Rentsch M, Seehofer D, Bruns CJ, Gebauer B, Modest HI, Held S, Folprecht G, Heinemann V, Neumann UP (2018) Surgical treatment options following chemotherapy plus cetuximab or bevacizumab in metastatic colorectal cancer-central evaluation of FIRE-3. Eur J Cancer 88:77–86. https://doi.org/10.1016/j.ejca.2017.10.028
Ohhara Y, Fukuda N, Takeuchi S, Honma R, Shimizu Y, Kinoshita I, Dosaka-Akita H (2016) Role of targeted therapy in metastatic colorectal cancer. World J Gastrointest Oncol 8(9):642–655. https://doi.org/10.4251/wjgo.v8.i9.642
Poston G, Adam R, Xu J, Byrne B, Esser R, Malik H, Wasan H, Xu J (2017) The role of cetuximab in converting initially unresectable colorectal cancer liver metastases for resection. Eur J Surg Oncol 43(11):2001–2011. https://doi.org/10.1016/j.ejso.2017.07.021
Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T, Hickish T, Butler R, Stanton L, Dixon E, Little L, Bowers M, Pugh S, Garden OJ, Cunningham D, Maughan T, Bridgewater J (2014) Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the new EPOC randomised controlled trial. Lancet Oncol 15(6):601–611. https://doi.org/10.1016/S1470-2045(14)70105-6
Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, Poston G, Bechstein W, Lentz MA, Mauer M, Folprecht G, Van Cutsem E, Ducreux M, Nordlinger B, European Organisation for R, Treatment of C, Gastro-Intestinal Tract Cancer G, Arbeitsgruppe Lebermetastasen und tumoren in der Chirurgischen Arbeitsgemeinschaft (2017) Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst 109(9):89. https://doi.org/10.1093/jnci/djx015
Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Giessen-Jung C, Moehler M, Jagenburg A, Kirchner T, Jung A, Heinemann V, Investigators F (2016) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 17(10):1426–1434. https://doi.org/10.1016/S1470-2045(16)30269-8
Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J et al (2015) Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 274(2):416–425
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96(4):261–268
Vatandoust S, Price TJ, Karapetis CS (2015) Colorectal cancer: metastases to a single organ. World J Gastroenterol 21(41):11767–11776. https://doi.org/10.3748/wjg.v21.i41.11767
Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O’Neil BH, Atkins JN, Berry S, Polite BN, O’Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB 3rd, Mulkerin DL, Mayer RJ, Blanke C (2017) Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA 317(23):2392–2401. https://doi.org/10.1001/jama.2017.7105
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, Ye QH, Yu Y, Xu B, Qin XY, Xu J (2013) Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol 31(16):1931–1938. https://doi.org/10.1200/JCO.2012.44.8308
Zellweger M, Abdelnour-Berchtold E, Krueger T, Ris HB, Perentes JY, Gonzalez M (2018) Surgical treatment of pulmonary metastasis in colorectal cancer patients: current practice and results. Crit Rev Oncol Hematol 127:105–116. https://doi.org/10.1016/j.critrevonc.2018.05.001
Acknowledgements
This research was supported by a Grant of the Seoul National University Bundang Hospital Research Grant (no. 14-2015-024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study protocol was reviewed and approved by the IRB of the Seoul National University Bundang Hospital (IRB Registration No: B-1805/466-177) and conducted in accordance with the precepts established by the Helsinki Declaration.
Informed consent
Informed consent for this study was waived due to the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, SA., Kim, JW., Suh, K.J. et al. Conversion surgery after cetuximab or bevacizumab plus FOLFIRI chemotherapy in colorectal cancer patients with liver- and/or lung-limited metastases. J Cancer Res Clin Oncol 146, 2399–2410 (2020). https://doi.org/10.1007/s00432-020-03233-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03233-7