Abstract
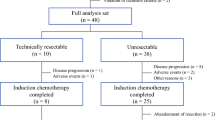
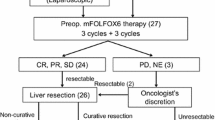
To investigate efficacy and safety of cetuximab combined with neo adjuvant chemotherapy regimen in patients with unresectable colorectal liver. This was a prospective trial with rate of Ro liver metastases resection as primary end point. Between January 2010 and December 2014, 46 patients with unresectable liver metastases from colon or rectum were enrolled. Patients received Cetuximab along with neoadjuvant chemotherapy where 34 (74 %) and 12 (2 6 %) patients received FOLFOX and FOLFIRI, respectively. They were assessed for response after 2–3 cycles by CT scan. Patients with resectable disease were offered liver surgery within 3–6 weeks of the last treatment cycle. The primary end point was resection rate of liver metastases, which was evaluated in all patients. Secondary end points were response rate according to Response evaluation criteria in solid tumors (RECIST) criteria, perioperative morbidity and mortality. An objective response was observed in 28 (60.9 %) patients. Seven (15.2 %) patients were reported radiologically to have a complete response (CR); 21 (45.7 %) patients had radiological partial response (PR). An additional 12 patients (26.1 %) demonstrated stable disease (SD) and only six patients (13.0 %) had disease progression (PD). Microscopically complete resections (R0 resection) was performed in all 28 patients (60.9 %). The most frequent toxicities were skin rash and diarrhoea. There was no operative mortality. Chemotherapy with cetuximab yields high response rates compared with historical controls, and leads to significantly increased resectability. Complete resection of previously unresectable colorectal liver metastases can be performed with minimal morbidity and mortality. This therapeutic strategy involves a multimodal approach.
Similar content being viewed by others
References
Landis SH, Murray T, Bolden S, Wingo PA (1999) Cancer statistics, 1999. CA Cancer J Clin 49(1):8–31
Williams NS, Northover JMA, Arnott SJ (1995) Colorectal tumors. In: Peckham M, Pinedo H, Veronesi U (eds) Oxford textbook of oncology. Oxford University Press, Oxford, pp 1133–1168
Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D (1993) Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 306(6880):752–755
Eberwein M., et. al (1998) Resection of hepatic metastases from colorectal cancer. Acta Chirurgica Austriaca 30(4):242–246
Adam R., et al. (2001) Five-year survival following hepatic resection after neoadjuvanttherapy for nonresectable colorectal. Ann Surg Oncol 8(4):347–353
Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L (1996) Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 224:509–520, discussion 520-522
Nordlinger B, Van Cutsem E, Rougier P et al (2007) Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer 43:2037–2045
Van Cutsem E, Nordlinger B, Adam R et al (2006) Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 42:2212–2221
Scheithauer W, Kornek GV, Raderer M, Schull B, Schmid K, Kovats E, Schneeweiss B, Lang F, Lenauer A, Depisch D (2003) Randomized multicenter phase ii trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 21(7):1307–1312
Masi G, Loupakis F, Pollina L, Vasile E, Cupini S, Ricci S, Brunetti IM, Ferraldeschi R, Naso G, Filipponi F, Pietrabissa A, Goletti O, Baldi G, Fornaro L, Andreuccetti M, Falcone A (2009) Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg 249:420–425
Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H (2004) Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg 240:1052–1061, discussion 1061-1064
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27(5):663–671
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, Konopke R, Stroszczynski C, Liersch T, Ockert D, Herrmann T, Goekkurt E, Parisi F, Köhne CH (2010) Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 11:38–47
Adam R, Aloia T, Lévi F, Wicherts DA, de Haas RJ, Paule B, Bralet MP, Bouchahda M, Machover D, Ducreux M, Castagne V, Azoulay D, Castaing D (2007) Hepatic resection after rescue cetuximab treatment for colorectal liver metastases previously refractory to conventional systemic therapy. J Clin Oncol 25:4593–4602
Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22(7):1201–1208
Van Cutsem E, Nowacki M, Lang S et al (2007) Randomized phase III study of irinotecan and 5FU/FA with or without cetuximab in the first line treatment of patients with colorectal cancer: the Crystal study. In: Proceedings of the American Society of Clinical Oncology (ASCO ‘07), vol. 25, p 164s, Chicago, Ill, USA, June 2007, abstract 4000
Garufi C, Torsello A, Tumulo S, Mottolese M, Campanella C, Zeuli M, Lo Re G, Pizzi G, Ettorre GM, Sperduti I (2009) POCHER (preoperative chemotherapy for hepatic resection) study with cetuximab (Cmab) plus CPT-11/5-fluorouracil (5-FU)/ leucovorin (FA)/oxaliplatin (L-OHP) (CPT-11-FFL) in unresectable colorectal liver metastases (CLM). J Clin Oncol 27:ae15020
De Roock W, Piessevaux H, De Schutter J et al (2008) KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 19:508–515
Bokemeyer C, Kohne C, Rougier P, Stroh C, Schlichting M, Van Cutsem E (2010) Cetuximab with chemotherapy (CT) as first-line treatment for metastatic colorectal cancer (mCRC): Analysis of the CRYSTAL and OPUS studies according to KRAS and BRAF mutation status. J Clin Oncol 28:a3506
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Benoist S, Brouquet A, Penna C, Julié C, El Hajjam M, Chagnon S, Mitry E, Rougier P, Nordlinger B (2006) Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 24:3939–3945
Taylor R, Fong Y (2007) Surgical treatment of hepatic metastases from colorectal cancer. In: Blumgart LH (ed) Surgery of the liver, biliary tract, and pancreas, 4th edn. Saunders Elsevier, Philadelphia, pp 1178–1194
Panis Y, Ribeiro J, Chrétien Y, Nordlinger B (1992) Dormant liver metastases: an experimental study. Br J Surg 79(3):221–223
Abdalla EK, Hicks ME, Vauthey JN (2001) Portal vein embolization: rationale, technique and future prospects. Br J Surg 88:165–175
Machi J, Uchida S, Sumida K, Limm WM, Hundahl SA, Oishi AJ, Furumoto NL, Oishi RH (2001) Ultrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg 5:477–489
Pérez–Soler R (2006) Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer 8(suppl 1):S7–S14
Pérez–Soler R, Van Cutsem E (2007) Clinical research of EGFR inhibitors and related dermatologic toxicities. Oncology 21(suppl 5):10–16
Sipples R (2006) Common side effects of anti-EGFR therapy: acneform rash. Semin Oncol Nurs 22(suppl 1):28–34
Sobrero AF, Maurel J, Fehrenbacher L et al (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26(14):2311–2319
Xiong HQ, Rosenberg A, LoBuglio A et al (2004) Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol 22(13):2610–2616
Lacouture ME, Cotliar J, Mitchell EP (2007) Clinical management ofcEGFRI dermatologic toxicities: U.S. perspective. Oncology 21(suppl 5):17–21
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Somashekhar, S.P., Ashwin, K.R., Zaveri, S.S. et al. Assessment of Tumor Response and Resection Rates in Unresectable Colorectal Liver Metastases Following Neoadjuvant Chemotherapy with Cetuximab. Indian J Surg Oncol 7, 11–17 (2016). https://doi.org/10.1007/s13193-015-0442-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-015-0442-8