Abstract
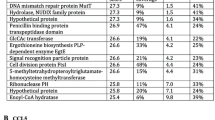
Mycobacteria encounter many different cells during infection within their hosts. Although alveolar epithelial cells play an essential role in host defense as the first cells to be challenged upon contact with mycobacteria, they may contribute to the acquisition of mycobacterial virulence by increasing the expression of virulence or adaptation factors prior to being ingested by macrophages on the side of pathogens. From this aspect, the enhanced virulence of nonpathogenic Mycobacterium smegmatis (MSM) passed through human alveolar A549 epithelial cells (A-MSM) was compared to the direct infection of MSM (D-MSM) in THP-1 macrophages and mouse models. The intracellular growth rate and cytotoxicity of A-MSM were significantly increased in THP-1 macrophages. In addition, compared to D-MSM, A-MSM induced relatively greater interleukin (IL)-1β, IL-6, IL-8, IL-12, TNF-α, MIP-1α, and MCP-1 in THP-1 macrophages. As a next step, a more persistent A-MSM infection was observed in a murine infection model with the development of granulomatous inflammation. Finally, 58 genes induced specifically in A-MSM were partially identified by differential expression using a customized amplification library. These gene expressions were simultaneously maintained in THP-1 infection but no changes were observed in D-MSM. Bioinformatic analysis revealed that these genes are involved mainly in bacterial metabolism including energy production and conversion, carbohydrate, amino acid, and lipid transport, and metabolisms. Conclusively, alveolar epithelial cells promoted the conversion of MSM to the virulent phenotype prior to encountering macrophages by activating the genes required for intracellular survival and presenting its pathogenicity.







Similar content being viewed by others
References
Smith DW, Wiegeshaus E, Navalkar R, Grover AA (1966) Host-parasite relationships in experimental airborne tuberculosis. I. Preliminary studies in BCG-vaccinated and nonvaccinated animals. J Bacteriol 91(2):718–724
Smith DW, Wiegeshaus EH (1989) What animal models can teach us about the pathogenesis of tuberculosis in humans. Rev Infect Dis 11(Suppl 2):S385–S393
Mendez-Samperio P, Trejo A, Perez A (2008) Mycobacterium bovis bacillus calmette-guerin (BCG) stimulates IL-10 production via the PI3K/Akt and p38 MAPK pathways in human lung epithelial cells. Cell Immunol 251(1):37–42
Sharma M, Sharma S, Roy S, Varma S, Bose M (2007) Pulmonary epithelial cells are a source of interferon-gamma in response to Mycobacterium tuberculosis infection. Immunol Cell Biol 85(3):229–237
Bermudez LE, Goodman J (1996) Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun 64(4):1400–1406
McDonough KA, Kress Y (1995) Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun 63(12):4802–4811
Garbe T, Harris D, Vordermeier M, Lathigra R, Ivanyi J, Young D (1993) Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun 61(1):260–267
Husson RN, James BE, Young RA (1990) Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol 172(2):519–524
Middleton AM, Chadwick MV, Nicholson AG, Dewar A, Feldman C, Wilson R (2003) Investigation of mycobacterial colonisation and invasion of the respiratory mucosa. Thorax 58(3):246–251
Garcia-Perez BE, Hernandez-Gonzalez JC, Garcia-Nieto S, Luna-Herrera J (2008) Internalization of a non-pathogenic mycobacteria by macropinocytosis in human alveolar epithelial A549 cells. Microb Pathog 45(1):1–6
Barker LP, Brooks DM, Small PL (1998) The identification of Mycobacterium marinum genes differentially expressed in macrophage phagosomes using promoter fusions to green fluorescent protein. Mol Microbiol 29(5):1167–1177
Dubnau E, Fontan P, Manganelli R, Soares-Appel S, Smith I (2002) Mycobacterium tuberculosis genes induced during infection of human macrophages. Infect Immun 70(6):2787–2795
Dubnau E, Smith I (2003) Mycobacterium tuberculosis gene expression in macrophages. Microbes Infect 5(7):629–637
Kim SY, Lee BS, Shin SJ, Kim HJ, Park JK (2008) Differentially expressed genes in Mycobacterium tuberculosis H37Rv under mild acidic and hypoxic conditions. J Med Microbiol 57(Pt 12):1473–1480
Alland D, Kramnik I, Weisbrod TR, Otsubo L, Cerny R, Miller LP, Jacobs WR Jr, Bloom BR (1998) Identification of differentially expressed mRNA in prokaryotic organisms by customized amplification libraries (DECAL): the effect of isoniazid on gene expression in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 95(22):13227–13232
Kim SY, Shin SJ, Song CH, Jo EK, Kim HJ, Park JK (2008) Identification of novel metronidazole-inducible genes in Mycobacterium smegmatis using a customized amplification library. FEMS Microbiol Lett 282(2):282–289
Bermudez LE, Sangari FJ, Kolonoski P, Petrofsky M, Goodman J (2002) The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect Immun 70(1):140–146
Sangari FJ, Goodman J, Bermudez LE (2000) Mycobacterium avium enters intestinal epithelial cells through the apical membrane, but not by the basolateral surface, activates small GTPase Rho and, once within epithelial cells, expresses an invasive phenotype. Cell Microbiol 2(6):561–568
Parker AE, Bermudez LE (1997) Expression of the green fluorescent protein (GFP) in Mycobacterium avium as a tool to study the interaction between mycobacteria and host cells. Microb Pathog 22(4):193–198
Shin SJ, Han JH, Manning EJ, Collins MT (2007) Rapid and reliable method for quantification of Mycobacterium paratuberculosis by use of the BACTEC MGIT 960 system. J Clin Microbiol 45(6):1941–1948
Lin Y, Zhang M, Barnes PF (1998) Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect Immun 66(3):1121–1126
Winter C, Taut K, Langer F, Mack M, Briles DE, Paton JC, Maus R, Srivastava M, Welte T, Maus UA (2007) FMS-like tyrosine kinase 3 ligand aggravates the lung inflammatory response to Streptococcus pneumoniae infection in mice: role of dendritic cells. J Immunol 179(5):3099–3108
Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, Glickman M, Jacobs WR Jr, Porcelli SA, Briken V (2007) Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog 3(7):e110
Crystal RJ (1991) Alveolar macrophages. In: Crystal RJ, West JB (eds) The lung: scientific foundations. Raven Press, New York, pp 527–538
Schneeberger EE (1991) Alveolar type II cells. In: Crystal RJ, West JB (eds) The lung: scientific foundations. Raven Press, New York, pp 736–758
Carbonara S, Tortoli E, Costa D, Monno L, Fiorentino G, Grimaldi A, Boscia D, Rollo MA, Pastore G, Angarano G (2000) Disseminated Mycobacterium terrae infection in a patient with advanced human immunodeficiency virus disease. Clin Infect Dis 30(5):831–835
Sawai T, Inoue Y, Doi S, Izumikawa K, Ohno H, Yanagihara K, Higashiyama Y, Miyazaki Y, Hirakata Y, Tashiro T, Kohno S (2006) A case of Mycobacterium nonchromogenicum pulmonary infection showing multiple nodular shadows in an immunocompetent patient. Diagn Microbiol Infect Dis 54(4):311–314
Sato K, Akaki T, Shimizu T, Sano C, Ogasawara K, Tomioka H (2001) Invasion and intracellular growth of Mycobacterium tuberculosis and Mycobacterium avium complex adapted to intramacrophagic environment within macrophages and type II alveolar epithelial cells. Kekkaku 76(2):53–57
Kaneko H, Yamada H, Mizuno S, Udagawa T, Kazumi Y, Sekikawa K, Sugawara I (1999) Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab Invest 79(4):379–386
Watford WT, Moriguchi M, Morinobu A, O’Shea JJ (2003) The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev 14(5):361–368
Abebe F, Bjune G (2006) The emergence of Beijing family genotypes of Mycobacterium tuberculosis and low-level protection by bacille calmette-guerin (BCG) vaccines: is there a link? Clin Exp Immunol 145(3):389–397
Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ (2006) Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol 79(1):80–86
Patel D, Danelishvili L, Yamazaki Y, Alonso M, Paustian ML, Bannantine JP, Meunier-Goddik L, Bermudez LE (2006) The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect Immun 74(5):2849–2855
Sander P, Rezwan M, Walker B, Rampini SK, Kroppenstedt RM, Ehlers S, Keller C, Keeble JR, Hagemeier M, Colston MJ, Springer B, Bottger EC (2004) Lipoprotein processing is required for virulence of Mycobacterium tuberculosis. Mol Microbiol 52(6):1543–1552
Rouquet G, Porcheron G, Barra C, Reperant M, Chanteloup NK, Schouler C, Gilot P (2009) A metabolic operon in extraintestinal pathogenic Escherichia coli promotes fitness under stressful conditions and invasion of eukaryotic cells. J Bacteriol 191(13):4427–4440
Sangari FJ, Seoane A, Rodriguez MC, Aguero J, Garcia Lobo JM (2007) Characterization of the urease operon of Brucella abortus and assessment of its role in virulence of the bacterium. Infect Immun 75(2):774–780
Zambelli B, Musiani F, Savini M, Tucker P, Ciurli S (2007) Biochemical studies on Mycobacterium tuberculosis UreG and comparative modeling reveal structural and functional conservation among the bacterial UreG family. Biochemistry 46(11):3171–3182
Bhave DP, Muse WB 3rd, Carroll KS (2007) Drug targets in mycobacterial sulfur metabolism. Infect Disord Drug Targets 7(2):140–158
Green J, Paget MS (2004) Bacterial redox sensors. Nat Rev Microbiol 2(12):954–966
Fisher MA, Plikaytis BB, Shinnick TM (2002) Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J Bacteriol 184(14):4025–4032
De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE III (2000) The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc Natl Acad Sci USA 97(3):1252–1257
Lagier B, Pelicic V, Lecossier D, Prod’hom G, Rauzier J, Guilhot C, Gicquel B, Hance AJ (1998) Identification of genetic loci implicated in the survival of Mycobacterium smegmatis in human mononuclear phagocytes. Mol Microbiol 29(2):465–475
Silver RF, Li Q, Ellner JJ (1998) Expression of virulence of Mycobacterium tuberculosis within human monocytes: virulence correlates with intracellular growth and induction of tumor necrosis factor alpha but not with evasion of lymphocyte-dependent monocyte effector functions. Infect Immun 66(3):1190–1199
Zhang M, Gong J, Lin Y, Barnes PF (1998) Growth of virulent and avirulent Mycobacterium tuberculosis strains in human macrophages. Infect Immun 66(2):794–799
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2010-0008728) and Basic Science Research Program through the NRF (R13-2007-020-00000-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Su-Young Kim, Hosung Sohn, and Go-Eun Choi contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
430_2011_190_MOESM1_ESM.ppt
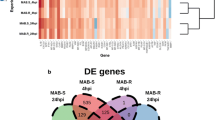
Supplementary Fig. 1 Spot density of dot blot hybridization of PCR probes with the Mycobacterium smegmatis library. Dot blots were hybridized with radio-labeled PCR probes under various conditions. Lane 1, M. smegmatis control; lane 2, M. smegmatis from THP-1; lane 3, M. smegmatis from A549; lane 4, M. smegmatis by passing A549 in THP-1 infection. The original results of dot blot hybridization are shown in the upper panel (a). The dot blot hybridization results were analyzed using ArrayGauge software. Spot density was indicated numerically and by color. The highest and lowest levels of expression are represented by red and blue, respectively (b). (PPT 869 kb)
Rights and permissions
About this article
Cite this article
Kim, SY., Sohn, H., Choi, GE. et al. Conversion of Mycobacterium smegmatis to a pathogenic phenotype via passage of epithelial cells during macrophage infection. Med Microbiol Immunol 200, 177–191 (2011). https://doi.org/10.1007/s00430-011-0190-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-011-0190-5