Abstract
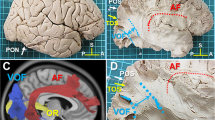
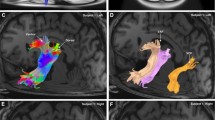
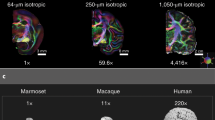
The primate brain contains a large number of interconnected visual areas, whose spatial organization and intracortical projections show a high level of conservation across species. One fiber pathway of recent interest is the vertical occipital fasciculus (VOF), which is thought to support communication between dorsal and ventral visual areas in the occipital lobe. A recent comparative diffusion MRI (dMRI) study reported that the VOF in the macaque brain bears a similar topology to that of the human, running superficial and roughly perpendicular to the optic radiation. The present study reports a comparative investigation of the VOF in the common marmoset, a small New World monkey whose lissencephalic brain is approximately tenfold smaller than the macaque and 150-fold smaller than the human. High-resolution ex vivo dMRI of two marmoset brains revealed an occipital white matter structure that closely resembles that of the larger primate species, with one notable difference. Namely, unlike in the macaque and the human, the VOF in the marmoset is spatially fused with other, more anterior vertical tracts, extending anteriorly between the parietal and temporal cortices. We compare several aspects of this continuous structure, which we term the VOF complex (VOF +), and neighboring fasciculi to those of macaques and humans. We hypothesize that the essential topology of the VOF+ is a conserved feature of the posterior cortex in anthropoid primates, with a clearer fragmentation into multiple named fasciculi in larger, more gyrified brains.





Similar content being viewed by others
References
Angelucci A, Rosa MGP (2015) Resolving the organization of the third tier visual cortex in primates: a hypothesis-based approach. Vis Neurosci 32:E010
Arcaro MJ, Livingstone MS (2017) Retinotopic organization of scene areas in macaque inferior temporal cortex. J Neurosci 37:7373–7389
Avants BB, Tustison NJ, Stauffer M et al (2014) The Insight ToolKit image registration framework. Front Neuroinform 8:44
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219
Brewer AA, Liu J, Wade AR, Wandell BA (2005) Visual field maps and stimulus selectivity in human ventral occipital cortex. Nat Neurosci 8:1102–1109
Budisavljevic S, Dell’Acqua F, Castiello U (2018) Cross-talk connections underlying dorsal and ventral stream integration during hand actions. Cortex 103:224–239
Bullock DN, Takemura H, Caiafa CF et al (2019) Associative white matter connecting the dorsal and ventral posterior human cortex. Brain Struct Funct 224:2631–2660
Caiafa CF, Pestilli F (2017) Multidimensional encoding of brain connectomes. Sci Rep 7:11491
Catani M, Howard RJ, Pajevic S, Jones DK (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94
Catani M, Jones DK, Ffytche DH (2005) Perisylvian language networks of the human brain. Ann Neurol 57:8–16
Catani M, Bodi I, Dell’Acqua F (2012) Comment on “The geometric structure of the brain fiber pathways”. Science 337:1605
Catani M, Robertsson N, Beyh A et al (2017) Short parietal lobe connections of the human and monkey brain. Cortex 97:339–357
Cavada C, Goldman-Rakic PS (1989) Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol 287:393–421
Clarke S (1994) Association and intrinsic connections of human extrastriate visual cortex. Proc Biol Sci 257:87–92
Croxson PL, Johansen-Berg H, Behrens TEJ et al (2005) Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci 25:8854–8866
Curran EJ (1909) A new association fiber tract in the cerebrum with remarks on the fiber tract dissection method of studying the brain. J Comp Neurol Psychol 19:645–656
Duan Y, Norcia AM, Yeatman JD, Mezer A (2015) The structural properties of major white matter tracts in strabismic amblyopia. Invest Ophthalmol Vis Sci 56:5152–5160
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25
Hashikawa T, Nakatomi R, Iriki A (2015) Current models of the marmoset brain. Neurosci Res 93:116–127
Jbabdi S, Lehman JF, Haber SN, Behrens TE (2013) Human and monkey ventral prefrontal fibers use the same organizational principles to reach their targets: tracing versus tractography. J Neurosci 33:3190–3201
Jeffs J, Federer F, Ichida JM, Angelucci A (2013) High-resolution mapping of anatomical connections in marmoset extrastriate cortex reveals a complete representation of the visual field bordering dorsal V2. Cereb Cortex 23:1126–1147
Jitsuishi T, Hirono S, Yamamoto T et al (2020) White matter dissection and structural connectivity of the human vertical occipital fasciculus to link vision-associated brain cortex. Sci Rep 10:820
Kaas JH (2013) The evolution of brains from early mammals to humans. WIREs Cogn Sci 4:33–45
Kamali A, Sair HI, Radmanesh A, Hasan KM (2014) Decoding the superior parietal lobule connections of the superior longitudinal fasciculus/arcuate fasciculus in the human brain. Neuroscience 277:577–583
Kolster H, Janssens T, Orban GA, Vanduffel W (2014) The retinotopic organization of macaque occipitotemporal cortex anterior to V4 and caudoventral to the middle temporal (MT) cluster. J Neurosci 34:10168–10191
Leopold DA, Mitchell JF, Freiwald WA (2017) Evolved mechanisms of high-level visual perception in primates. In: Kaas J (ed) Evolution of nervous systems, 2nd edition. pp 203–235
Lerma-Usabiaga G, Carreiras M, Paz-Alonso PM (2018) Converging evidence for functional and structural segregation within the left ventral occipitotemporal cortex in reading. Proc Natl Acad Sci USA 115:E9981–E9990
Levin N, Dumoulin SO, Winawer J et al (2010) Cortical maps and white matter tracts following long period of visual deprivation and retinal image restoration. Neuron 65:21–31
Liu C, Ye FQ, Newman JD et al (2020) A resource for the detailed 3D mapping of white matter pathways in the marmoset brain. Nat Neurosci 23:271–280
Lyon DC, Connolly JD (2012) The case for primate V3. Proc Biol Sci 279:625–633
Makris N, Papadimitriou GM, Kaiser JR et al (2009) Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 19:777–785
Makris N, Zhu A, Papadimitriou GM et al (2017) Mapping temporo-parietal and temporo-occipital cortico-cortical connections of the human middle longitudinal fascicle in subject-specific, probabilistic, and stereotaxic Talairach spaces. Brain Imaging Behav 11:1258–1277
Maldonado IL, Menjot de Champfleur N, Velut S et al (2013) Evidence of a middle longitudinal fasciculus in the human brain from fiber dissection. J Anat 223:38–45
Mars RB, Foxley S, Verhagen L et al (2016) The extreme capsule fiber complex in humans and macaque monkeys: a comparative diffusion MRI tractography study. Brain Struct Funct 221:4059–4071
Martino J, Garcia-Porrero JA (2013) In reply: wernicke’s perpendicular fasciculus and vertical portion of the superior longitudinal fasciculus. Neurosurgery 73:E382–E383
Menjot de Champfleur NM, Maldonado IL, Moritz-Gasser S et al (2013) Middle longitudinal fasciculus delineation within language pathways: a diffusion tensor imaging study in human. Eur J Radiol 82:151–157
Miller KL, Stagg CJ, Douaud G et al (2011) Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage 57:167–181
Miller KL, McNab JA, Jbabdi S, Douaud G (2012) Diffusion tractography of post-mortem human brains: optimization and comparison of spin echo and steady-state free precession techniques. Neuroimage 59:2284–2297
Mitchell JF, Leopold DA (2015) The marmoset monkey as a model for visual neuroscience. Neurosci Res 93:20–46
Morel A, Bullier J (1990) Anatomical segregation of two cortical visual pathways in the macaque monkey. Visual Neurosci 4:555–578
Ogawa S, Takemura H, Horiguchi H et al (2014) White matter consequences of retinal receptor and ganglion cell damage. Invest Ophthalmol Vis Sci 55:6976–6986
Pajevic S, Pierpaoli C (1999) Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn Reson Med 42:526–540
Panesar SS, Belo JTA, Yeh F-C, Fernandez-Miranda JC (2019) Structure, asymmetry, and connectivity of the human temporo-parietal aslant and vertical occipital fasciculi. Brain Struct Funct 224:907–923
Pestilli F, Yeatman JD, Rokem A et al (2014) Evaluation and statistical inference for human connectomes. Nat Methods 11:1058–1063
Petr R, Holden LB, Jirout J (1949) The efferent intercortical connections of the superficial cortex of the temporal lobe (Macaca mulatta). J Neuropathol Exp Neurol 8:100–103
Pierpaoli C, Walker L, Irfanoglu MO et al (2010) TORTOISE: an integrated software package for processing of diffusion MRI data. In: ISMRM 18th annual meeting. Stockholm, Sweden
Press WA, Brewer AA, Dougherty RF et al (2001) Visual areas and spatial summation in human visual cortex. Vision Res 41:1321–1332
Rademacher J, Caviness VS Jr, Steinmetz H, Galaburda AM (1993) Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex 3:313–329
Reveley C, Seth AK, Pierpaoli C et al (2015) Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc Natl Acad Sci USA 112:E2820–E2828
Reveley C, Gruslys A, Ye FQ et al (2016) Three-dimensional digital template atlas of the macaque brain. Cereb Cortex 27:4463–4477
Rilling JK, Glasser MF, Preuss TM et al (2008) The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci 11:426–428
Roebroeck A, Galuske R, Formisano E et al (2008) High-resolution diffusion tensor imaging and tractography of the human optic chiasm at 9.4 T. Neuroimage 39:157–168
Rogers J, Kochunov P, Zilles K et al (2010) On the genetic architecture of cortical folding and brain volume in primates. Neuroimage 53:1103–1108
Rokem A, Takemura H, Bock AS et al (2017) The visual white matter: the application of diffusion MRI and fiber tractography to vision science. J Vis 17(2):4
Rosa MGP, Tweedale R (2005) Brain maps, great and small: lessons from comparative studies of primate visual cortical organization. Philos Trans R Soc Lond B Biol Sci 360:665–691
Rosa MGP, Palmer SM, Gamberini M et al (2009) Connections of the dorsomedial visual area: pathways for early integration of dorsal and ventral streams in extrastriate cortex. J Neurosci 29:4548–4563
Sani I, McPherson BC, Stemmann H et al (2019) Functionally defined white matter of the macaque monkey brain reveals a dorso-ventral attention network. Elife 8:e40520
Schaeffer DJ, Adam R, Gilbert KM et al (2017) Diffusion-weighted tractography in the common marmoset monkey at 9.4T. J Neurophysiol 118:1344–1354
Schmahmann JD, Pandya D (2006) Fiber pathways of the brain. Oxford Univ Press, New York
Schmahmann JD, Pandya DN, Wang R et al (2007) Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653
Schurr R, Filo S, Mezer AA (2019) Tractography delineation of the vertical occipital fasciculus using quantitative T1 mapping. NeuroImage 202:116121
Seltzer B, Pandya DN (1984) Further observations on parieto-temporal connections in the rhesus monkey. Exp Brain Res 55:301–312
Smith AT, Greenlee MW, Singh KD et al (1998) The processing of first- and second-order motion in human visual cortex assessed by functional magnetic resonance imaging (fMRI). J Neurosci 18:3816–3830
Solomon SG, Rosa MGP (2014) A simpler primate brain: the visual system of the marmoset monkey. Front Neural Circuits 8:96
Sotiropoulos SN, Jbabdi S, Xu J et al (2013) Advances in diffusion MRI acquisition and processing in the human Connectome project. Neuroimage 80:125–143
Sun SW, Neil JJ, Song SK (2003) Relative indices of water diffusion anisotropy are equivalent in live and formalin-fixed mouse brains. Magn Reson Med 50:743–748
Takemura H, Caiafa CF, Wandell BA, Pestilli F (2016a) Ensemble tractography. PLoS Comput Biol 12:e1004692
Takemura H, Rokem A, Winawer J et al (2016b) A major human white-matter pathway between dorsal and ventral visual cortex. Cereb Cortex 26:2205–2214
Takemura H, Pestilli F, Weiner KS et al (2017) Occipital white matter tracts in human and macaque. Cereb Cortex 27:3346–3359
Takemura H, Pestilli F, Weiner KS (2019) Comparative neuroanatomy: integrating classic and modern methods to understand association fibers connecting dorsal and ventral visual cortex. Neurosci Res 146:1–12
Tax CMW, Dela Haije T, Fuster A et al (2016) Sheet Probability Index (SPI): characterizing the geometrical organization of the white matter with diffusion MRI. Neuroimage 142:260–279
Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ et al (2011) A lateralized brain network for visuospatial attention. Nat Neurosci 14:1245–1246
Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M (2012) Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48:82–96
Thiebaut de Schotten M, Cohen L, Amemiya E et al (2014) Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex 24:989–995
Thiebaut de Schotten M, Croxson PL, Mars RB (2019) Large-scale comparative neuroimaging: Where are we and what do we need? Cortex 118:188–202
Thomas C, Ye FQ, Irfanoglu MO et al (2014) Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci USA 111:46
Tootell RB, Tsao D, Vanduffel W (2003) Neuroimaging weighs in humans meet macaques in “primate” visual cortex. J Neurosci 23:3981–3989
Tournier JD, Calamante F, Connelly A (2012) MRtrix: diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol 22:53–66
Ungerleider LG, Mishkin M (1982) Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW (eds) The analysis of visual behavior. MIT Press, Cambridge, pp 549–586
Ungerleider LG, Galkin TW, Desimone R, Gattass R (2008) Cortical connections of area V4 in the macaque. Cereb Cortex 18:477–499
Van Essen DC, Glasser MF (2018) Parcellating cerebral cortex: how invasive animal studies inform noninvasive mapmaking in humans. Neuron 99:640–663
Van Essen DC, Smith SM, Barch DM et al (2013) The WU-Minn human connectome project: an overview. Neuroimage 80:62–79
Vogt O (1904) Neurobiologische Arbeiten. Fischer
Wade AR, Brewer AA, Rieger JW, Wandell BA (2002) Functional measurements of human ventral occipital cortex: retinotopy and colour. Philos Trans R Soc Lond B Biol Sci 357:963–973
Wakana S, Jiang H, Nagae-Poetscher LM et al (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 230:77–87
Wallisch P, Movshon JA (2008) Structure and function come unglued in the visual cortex. Neuron 60:195–197
Wandell BA, Winawer J (2011) Imaging retinotopic maps in the human brain. Vision Res 51:718–737
Wang Y, Fernández-Miranda JC, Verstynen T et al (2013) Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cereb Cortex 23:2347–2356
Wedeen VJ, Rosene DL, Wang R et al (2012) The geometric structure of the brain fiber pathways. Science 335:1628–1634
Weiner KS, Yeatman JD, Wandell BA (2017) The posterior arcuate fasciculus and the vertical occipital fasciculus. Cortex 97:274–276
Winawer J, Horiguchi H, Sayres RA et al (2010) Mapping hV4 and ventral occipital cortex: the venous eclipse. J Vis 10:1
Woodward A, Hashikawa T, Maeda M et al (2018) The Brain/MINDS 3D digital marmoset brain atlas. Sci Data 5:180009
Wu Y, Sun D, Wang Y et al (2016) Tracing short connections of the temporo-parieto-occipital region in the human brain using diffusion spectrum imaging and fiber dissection. Brain Res 1646:152–159
Yeatman JD, Dougherty RF, Myall NJ et al (2012) Tract profiles of white matter properties: automating fiber-tract quantification. PLoS ONE 7:e49790
Yeatman JD, Rauschecker AM, Wandell BA (2013) Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang 125:146–155
Yeatman JD, Weiner KS, Pestilli F et al (2014) The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proc Natl Acad Sci USA 111:E5214–E5223
Yoshimine S, Ogawa S, Horiguchi H et al (2018) Age-related macular degeneration affects the optic radiation white matter projecting to locations of retinal damage. Brain Struct Funct 223:3889–3900
Zhu Q, Vanduffel W (2019) Submillimeter fMRI reveals a layout of dorsal visual cortex in macaques, remarkably similar to New World monkeys. Proc Natl Acad Sci USA 116:2306–2311
Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H (1989) Gyrification in the cerebral cortex of primates. Brain Behav Evol 34:143–150
Zilles K, Palomero-Gallagher N, Amunts K (2013) Development of cortical folding during evolution and ontogeny. Trends Neurosci 36:275–284
Acknowledgements
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (JP15K16015 and JP19K20653, T.K; JP15J00412 and JP17H04684, H.T), the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Japan Agency for Medical Research and Development (Grant no. JP19dm0207001). D.A.L. and F.Q.Y. are supported by the Intramural Research Program of the National Institute of Mental Health (ZIC MH002899). F.P. was supported by NSF IIS-1636893, NSF BCS-1734853, NSF IIS-1912270 and NSF AOC 1916518, a Microsoft Investigator Fellowship. A.C.S. was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (ZIA NS003041). Human dMRI data used to produce the figure in this article were provided by the Human Connectome Project, WU-Minn Consortium (Van Essen, D. and Ugürbil, K., 1U54MH091657).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests associated with this article.
Research involving human participants
Data collection from human participants was approved by the Institutional Review Boards (IRBs) of the University of Washington, Saint Louis (HCP data set).
Informed consent
All participants provided written informed consent to participate in the project.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaneko, T., Takemura, H., Pestilli, F. et al. Spatial organization of occipital white matter tracts in the common marmoset. Brain Struct Funct 225, 1313–1326 (2020). https://doi.org/10.1007/s00429-020-02060-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-020-02060-3