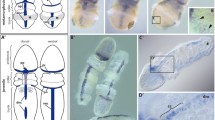
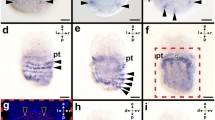
Abstract
Members of the orthodenticle (otd/Otx) and empty spiracles (ems/Emx) gene families are head gap genes that encode homeodomain-containing DNA-binding proteins. Although numerous studies show their central role in developmental processes in brain specification, a surprisingly high number of other developmental processes have been shown to involve their expression. In this paper, we report the identification and expression of ems and otd in two chelicerate species: a scorpion, Euscorpius flavicaudis (Chactidae, Scorpiona, Arachnida, Euchelicerata) and a spider, Tegenaria saeva (Aranea, Arachnida, Euchelicerata). We show that both ems and otd are expressed not only in an anterior head domain but also along the entire anterior–posterior axis during embryonic development. The expression patterns for both genes are typically segmental and concern neurectodermal territories. During patterning of the opisthosoma, ems and otd are expressed in the lateral ectoderm just anterior to the limb bud primordia giving rise to respiratory organs and spinnerets (spider). This common pattern found in two divergent species thus appears to be a conserved character of chelicerates. These results are discussed in terms of evolutionary origin of respiratory organs and/or functional pathway recruitment.







Similar content being viewed by others
References
Akiyama-Oda Y, Oda H (2003) Early patterning of the spider embryo: a cluster of mesenchymal cells at the cumulus produces Dpp signals received by germ disc epithelial cells. Development 130:1735–1757
Anderson DT (1973) Embryology and phylogeny in annelids and arthropods. Pergamon, New York
Brauer A (1895) Beiträge zur Kenntnis der Entwicklungsgeschichte des Skorpions, II. Z Wiss Zool 59:351–435
Cohen SM, Jürgens G (1990) Mediation of Drosophila head development by gap-like segmentation genes. Nature 346:482–485
Cohen S, Jürgens G (1991) Drosophila headlines. Trends Genet 7:267–272
Dalton D, Chadwick R, McGinnis W (1989) Expression and embryonic function of empty spiracles: a Drosophila homeobox gene with two patterning functions on the anterior–posterior axis of the embryo. Genes Dev 3:1940–1956
Damen WGM (2002) Parasegmental organization of the spider embryo implies that the parasegment is an evolutionary conserved entity in arthropod embryogenesis. Development 129:1239–1250
Damen WMG, Saridaki T, Averof M (2002) Diverse adaptations of an ancestral gill: a common evolutionary origin for wings, breathing organs, and spinnerets. Curr Biol 12:1711–1716
Deutsch J (2004) Segments and parasegments in Arthropods: a functional perspective. Bioessays 26:1117–1125
Dunlop JA (1996) Systematics of the fossils arachnids. Rev Suisse Zool Vol hors série 173–184
Ebner A, Kiefer FN, Ribeiro C, Petit V, Nussbaumer, Affolter M (2002) Tracheal development in Drosophila melanogaster as a model system for studying the development of a branched organ. Gene 287:55–66
Farley RD (2001) Abdominal plates, spiracles and sternites in the ventral mesosoma of embryos of the desert scorpion Paructonus mesaensis (Scorpiones: Vaejovidae). Invertebr Reprod Dev 40:193–208
Farley RD (2005) Developmental changes in the embryo, protonymph and first molt of the scorpion Centruroides vittatus (Scorpiones: Buthidae). J Morphol 265:1–27
Finkelstein R, Perrimon N (1991) The molecular genetics of head development in Drosophila melanogaster. Development 112:899–912
Finkelstein R, Smouse D, Capaci TM, Spradling AC, Perrimon N (1990) The orthodenticle gene encodes a novel homeodomain protein involved in the development of the Drosophila nervous system and ocellar visual structures. Genes Dev 4:1516–1527
Galliot B, de Vargas C, Miller D (1999) Evolution of homeobox genes: Q50 Paired-like genes founded the Paired class. Dev Genes Evol 209:186–197
Gallitano-Mendel A, Finkelstein R (1998) Ectopic orthodenticle expression alters segment polarity gene expression but not head segment identity in the Drosophila embryo. Dev Biol 199:125–137
Gauchat D, Mazet F, Berney C, Schummer M, Kreger S, Pawlowski J, Galliot B (2000) Evolution of Antp-class genes and differential expression of Hydra Hox/paraHox genes in anterior patterning. Proc Natl Acad Sci USA 97:4493–4498
Gibert JM, Mouchel-Vielh E, Queinnec E, Deutsch JS (2000) Barnacle duplicate engrailed genes: divergent expression patterns and evidence for a vestigial abdomen. Evol Dev 2:194–202
Grossniklaus U, Cadigan KM, Gehring WJ (1991) Three maternal coordinate systems cooperate in the patterning of the Drosophila head. Development 120:3155–3171
Hartmann B, Hirth F, Walldorf U, Reichert H (2000) Expression, regulation and function of the homeobox gene empty spiracles in brain and ventral nerve cord development of Drosophila. Mech Dev 90:143–153
Hirth F, Therianos S, Loop T, Gehring WJ, Reichert H, Furukubo-Tokunaga K (1995) Developmental defects in brain segmentation caused by mutations of the homeobox genes orthodenticle and empty spiracles in Drosophila. Neuron 15:769–778
Hu N, Castelli-Gair J (1999) Study of the posterior spiracles of Drosophila as a model to understand the genetic and cellular mechanisms controlling morphogenesis. Dev Biol 214:197–210
Hughes CL, Kaufman TC (2002) Exploring myriapod segmentation: the expression patterns of even-skipped, engrailed, and wingless in a centipede. Dev Biol 247:47–61
Lall S, Patel NH (2001) Conservation and divergence in molecular mechanisms of axis formation. Annu Rev Genet 35:407–437
Li Y, Brown SJ, Hausdorf B, Tautz D, Denell RE, Finkelstein R (1996) Two orthodenticle-related genes in the short-germ beetle Tribolium castaneum. Dev Genes Evol 206:35–45
Lichtneckert R, Reichert H (2005) Insights into the urbilaterian brain: conserved genetic patterning mechanisms in insect and vertebrate brain development. Heredity 94:465–477
Lynch JA, Brent AE, Leaf DS, Pultz MA, Desplan C (2006) Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature 439:728–732
Martinez-Arias A, Lawrence PA (1985) Parasegments and compartments in the Drosophila embryo. Nature 313:639–642
McClendon JF (1905) On the anatomy and embryology of the nervous system of the scorpion. Biol Bull 8:38–55
Mittmann B, Scholtz G (2001) Distal-less expression in embryos of Limulus polyphemus (Chelicerata, Xiphosura) and Lepisma saccharina (Insecta, Zygentoma) suggests as role in the development of mechanoreceptors, chemoreceptors, and the CNS. Dev Genes Evol 211:232–243
Müller P, Yanze N, Schmid V, Spring J (1999) The homeobox gene otx of the jellyfish Podocoryne carnea: role of a head gene in striated muscle and evolution. Dev Biol 216:582–594
Root TM (1990) Neurobiology. In: Polis GA (ed) The biology of scorpions. Stanford University Press, Stanford, pp 341–413
Royet J, Finkelstein R (1995) Pattern formation in Drosophila head development: the role of the orthodenticle homeobox gene. Development 121(11):3561–3572
Schimkewitsch W (1887) Etude sur le développement des araignées. Arch Biol 6:515–584
Scholtz G, Kamenz C (2006) The book lungs of Scorpiones and Tetrapulmonata (Chelicerata, Arachnida): evidence for homology and a single terrestrialisation event of a common arachnid ancestor. Zoology 109:2–13
Schröder R (2003) The genes orthodenticle and hunchback substitute for bicoid in the beetle Tribolium. Nature 422:621–625
Simonnet F, Deutsch J, Quéinnec E (2004) hedgehog is a segment polarity gene in a crustacean and a chelicerate. Dev Genes Evol 214:537–545
Stollewerk A (2002) Recruitment of cell groups through Delta/Notch signalling during spider neurogenesis. Development 129:5339–5348
Stollewerk A, Weller M, Tautz D (2001) Neurogenesis in the spider Cupiennius salei. Development 128:2673–2688
Stollewerk A, Tautz D, Weller M (2003) Neurogenesis in the spider: new insights from comparative analysis of morphological processes and gene expression patterns. Arthropod Struct Dev 32:5–16
Telford MJ, Thomas RH (1998) Expression of homeobox genes shows chelicerate arthropods retain their deutocerebral segment. Proc Natl Acad Sci U S A 95:10671–10675
Walldorf L, Gehring WJ (1992) Empty spiracles, a gap gene containing a homeobox involved in Drosophila head development. EMBO J 11:2247–2259
Weygoldt P, Paulus HF (1979) Untersuchungen zur Morphologie, Taxonomie und Phylogenie der Chelicerata. Z Zoolog Syst Evol-forsch 17:85–116, 177–200
Wimmer EA, Cohen SM, Jackle H, Desplan C (1997) Buttonhead does not contribute to a combinatorial code proposed for Drosophila head development. Development 124:1509–1517
Acknowledgements
We are particularly grateful to Gerhard Scholtz for his thorough reading of the manuscript and for his constructive comments and suggestions. We are grateful to Roland Stockmann for providing scorpions and scanning electron micrographs and to Pierrette Lamarre and Murielle Jager for their technical help during this work. We thank Jason Dunlop for comments on a first draft of the paper and Jean Deutsch and Michael Manuel for critical reading of the manuscript. Many thanks to David Cribbs for help in English writing. We are indebted to the two referees for critically reading and mostly improving the manuscript. F.S. is the recipient of a Ph.D. fellowship from the Ministère de la Recherche et de la Technologie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by guest editors Jean Deutsch and Gerhard Scholtz
Rights and permissions
About this article
Cite this article
Simonnet, F., Célérier, ML. & Quéinnec, E. Orthodenticle and empty spiracles genes are expressed in a segmental pattern in chelicerates. Dev Genes Evol 216, 467–480 (2006). https://doi.org/10.1007/s00427-006-0093-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-006-0093-4