Abstract
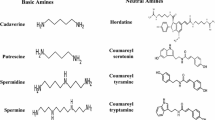
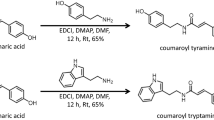
Hydroxycinnamic acid amides (HCAAs) are secondary metabolites involved in the defense of plants against pathogens. Here, we report the first identification of HCAAs, p-coumaroylagmatine, feruloylagmatine, p-coumaroylputrescine and feruloylputrescine, in Arabidopsis thaliana rosette leaves infected with Alternaria brassicicola and the assignment of At5g61160 as the agmatine coumaroyltransferase (AtACT) that catalyzes the last reaction in the biosynthesis of the HCAAs. Feeding experiments with putative labeled precursors revealed that the four HCAAs were synthesized from hydroxycinnamic acids and agmatine or putrescine. AtACT gene function was identified from an analysis of a mutant that did not accumulate HCAAs. In wild-type Arabidopsis, AtACT transcripts markedly increased in response to A. brassicicola infection. Enzymatic activity that catalyzes the synthesis of the HCAAs was confirmed in vitro by using a recombinant AtACT expressed in Escherichia coli. The Atact mutant was susceptible to infection by A. brassicicola, indicating that HCAAs are responsible for defense against pathogens in A. thaliana.







Similar content being viewed by others
Abbreviations
- HCAA:
-
Hydroxycinnamic acid amide
- CouAgm:
-
p-Coumaroylagmatine
- FerAgm:
-
Feruloylagmatine
- CouPtr:
-
p-Coumaroylputrescine
- FerPtr:
-
Feruloylputrescine
- ACT:
-
Agmatine coumaroyltransferase
- AtACT:
-
Arabidopsis thaliana agmatine coumaroyltransferase
References
Alcázar R, García-Martínez JL, Cuevas JC, Tiburcio AF, Altabella T (2005) Overexpression of ADC2 in Arabidopsis induces dwarfism and late-flowering through GA deficiency. Plant J 45:425–436
Bernards M, Lopez ML, Zajicek J, Lewis NG (1995) Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin. J Biol Chem 270:7382–7386
Burhenne K, Kristensen BK, Rasmussen SK (2003) A new class of N-hydroxycinnamoyltransferase. J Biol Chem 278:13919–13927
D’Auria JC (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9:331–340
Fellenberg C, Milkowski C, Hause B, Lange PR, Böttcher C, Schmidt J, Vogt T (2008) Tapetum-specific location of a cation-dependent O-methyltransferase in Arabidopsis thaliana. Plant J 56:132–145
Fester T, Maier W, Strack D (1999) Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co-inoculation with rhizosphere bacteria. Mycorrhiza 8:241–246
Fujiwara H, Tanaka Y, Yonekura-Sakakibara K, Fukuchi-Mizutani M, Nakao M, Fukui Y, Yamaguchi M, Ashikari T, Kusumi T (1998) cDNA cloning, gene expression and subcellular localization of anthocyanin 5-aromatic acyltransferase from Gentiana triflora. Plant J 16:421–431
Grienenberger E, Besseau S, Geoffroy P, Debayle D, Heintz D, Lapierre C, Pollet B, Heitz T, Legrand M (2009) A BAHD acyltransferase is expressed in the tapetum of Arabidopsis anthers and is involved in the synthesis of hydroxycinnamoyl spermidines. Plant J doi:10.1111/j.1365-313X.2008.03773.x
Ishihara A, Hashimoto Y, Tanaka C, Dubouzet JG, Nakao T, Matsuda F, Nishioka T, Miyagawa H, Wakasa K (2008) The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J 54:481–495
Jin S, Yoshida M, Nakajima T, Murai A (2003) Accumulation of hydroxycinnamic acid amides in winter wheat under snow. Biosci Biotechnol Biochem 67:1245–1249
Keller H, Hohlfeld H, Wray V, Hahlbrock K, Scheel D, Strack D (1996) Changes in the accumulation of soluble and cell wall-bound phenolics in elicitor-treated cell suspension cultures and fungus-infected leaves of Solanum tuberosum. Phytochemistry 42:389–396
Martin-Tanguy J, Martin C, Gallet M, Vernoy R (1976) Potent natural inhibitors of tobacco mosaic virus multiplication. C R Acad Sci Hebd Seances Acad Sci D 282:2231–2234
Martin-Tanguy J, Deshayes A, Perdrizet E, Martin C (1979) Hydroxycinnamic acid amides (HCA) in Zea mays. FEBS Lett 108:176–178
Matsuda F, Morino K, Ano R, Kuzawa M, Wakasa K, Miyagawa H (2005) Metabolic flux analysis of the phenylpropanoid pathway in elicitor-treated potato tuber tissue. Plant Cell Physiol 46:454–466
Mayama S, Tani T, Matauura Y, Ueno T, Fukami H (1981) The production of phytoalexins by oat in response to crown rust, Puccinia coronata f. sp. avenae. Physiol Plant Pathol 19:217–226
Mayama S, Matsuura Y, Iida H, Tani T (1982) The role of avenalumin in the resistance of oat to crown rust, Puccinia coronata f. sp. avenae. Physiol Plant Pathol 20:189–199
Miyagawa H, Ishihara A, Nishimoto T, Ueno T, Mayama S (1995) Induction of avenanthramides in oat leaves inoculated with crown rust fungus, Puccinia coronata f. sp. avenae. Biosci Biotechnol Biochem 59:2305–2306
Negrel J, Jeandet P (1987) Metabolism of tyramine and feruloyltyramine in TMV inoculated leaves of Nicotiana tabacum. Phytochemistry 26:2185–2190
Negrel J, Paynot M, Javelle F (1992) Purification and properties of putrescine hydroxycinnamoyl transferase from tobacco (Nicotiana tabacum) cell suspensions. Plant Physiol 98:1264–1269
Nomura T, Ishizuka A, Kishida K, Islam AKMR, Endo TR, Iwamura H, Ishihara A (2007) Chromosome arm location of the genes for the biosynthesis of hordatines in barley. Genes Genet Syst 82:455–464
Ogura Y, Ishihara A, Iwamura H (2001) Induction of hydroxycinnamic acid amides and tryptophan by jasmonic acid, abscisic acid and osmotic stress in barley leaves. Z Naturforsch 56c:193–202
Okazaki Y, Isobe T, Iwata Y, Matsukawa T, Matsuda F, Miyagawa H, Ishihara A, Nishioka T, Iwamura H (2004) Metabolism of avenanthramide phytoalexins in oats. Plant J 39:560–572
Rowe HC, Kliebenstein DJ (2008) Complex genetics control natural variation in Arabidopsis thaliana resistance to Botrytis cinerea. Genetics 180:2237–2250
Schmidt A, Scheel D, Strack D (1998) Elicitor-stimulated biosynthesis of hydroxycinnamoyltyramines in cell suspension cultures of Solanum tuberosum. Planta 205:51–55
Schmidt A, Grimm R, Schmidt J, Scheel D, Strack D, Rosahl S (1999) Cloning and expression of a potato cDNA encoding hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase. J Biol Chem 274:4273–4280
Soylu S (2006) Accumulation of cell-wall bound phenolic compounds and phytoalexin in Arabidopsis thaliana leaves following inoculation with pathovars of Pseudomonas syringae. Plant Sci 170:942–952
Stoessl A (1967) The antifungal factors in barley. IV. Isolation, structure, and synthesis of the hordatines. Can J Chem 45:1745–1760
Suzuki H, Nishino T, Nakayama T (2007) cDNA cloning of a BAHD acyltransferase from soybean (Glycine max): isoflavone 7-O-glucoside-6″-O-malonyltransferase. Phytochemistry 68:2035–2042
Thomma BPHJ, Nelissen I, Eggermont K, Broekaert WF (1999) Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J 19:163–171
Tsuji J, Jackson EP, Gage DA, Hammerschmidt R, Somerville SC (1992) Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas syringae pv syringae. Plant Physiol 98:1304–1309
Ueda M, Tashiro C, Yamamura S (1997) cis-p-Coumaroylagmatine, the genuine leaf-opening substance of nyctinastic plant, Albizzia julibrissin Durazz. Tetrahedron Lett 38:3253–3256
Urano K, Hobo T, Shinozaki K (2005) Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed development. FEBS Lett 579:1557–1564
von Röpenack E, Parr A, Schulze-Lefert P (1998) Structural analyses and dynamics of soluble and cell wall-bound phenolics in a broad spectrum resistance to the powdery mildew fungus in barley. J Biol Chem 273:9013–9022
Yang Q, Reinhard K, Schilz E, Matern U (1997) Characterization and heterologous expression of hydroxycinamoyl/benzoyl-CoA:anthranilate N-hydroxycinnamoyl/benzoyltransferase from elicited cell cultures of carnation, Dianthus caryophyllus L. Plant Mol Biol 35:777–789
Yang Q, Trinh HX, Imai S, Ishihara A, Zhang L, Nakayashiki H, Tosa Y, Mayama S (2004) Analysis of the involvement of hydroxyanthranilate hydroxycinnamoyltransferase and caffeoyl-CoA 3-O-methyltransferase in phytoalexin biosynthesis in oat. Mol Plant Microbe Interact 17:81–89
Acknowledgments
The authors are grateful to the Radioisotope Research Center, Kyoto University, for instrumental support with the radioisotope experiments. This work was partly supported by Grants-in-aid for Scientific Research (No. 18380066 to T.N. and No. 21580126 to A.I.) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Muroi, A., Ishihara, A., Tanaka, C. et al. Accumulation of hydroxycinnamic acid amides induced by pathogen infection and identification of agmatine coumaroyltransferase in Arabidopsis thaliana . Planta 230, 517–527 (2009). https://doi.org/10.1007/s00425-009-0960-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-0960-0