Abstract
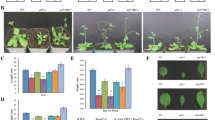
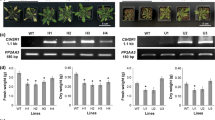
A panel of aluminum-tolerant (AlRes) mutants was isolated by protoplast-based T-DNA activation tagging in the tobacco cultivar SR1. The mutants fell into two phenotypic classes: a minority of the mutants were fertile and developed similarly to the wild type (type I), the majority was male-sterile and grew as semi-dwarfs (type II). These traits, along with the aluminum tolerance, were inherited in a monogenic dominant manner. Both types of mutants were characterized by excessive bundling of actin microfilaments and by a strongly increased abundance of actin, a phenotype that could be partially phenocopied in the wild type by treatment with aluminum chloride. The actin bundles could be dissociated into finer strands by addition of exogenous auxin in both types of mutants. However, actin microfilaments and leaf expansion were sensitive to blockers of actin assembly in the wild type and in the mutants of type I, whereas they were more tolerant in the mutants of type II. The mutants of type II displayed a hypertrophic development of vasculature, manifest in form of supernumerary leaf veins and extended xylem layers in stems and petioles. Whereas mutants of type I were characterized by a normal, but aluminum-tolerant polar auxin-transport, auxin-transport was strongly promoted in the mutants of type II. The phenotype of these mutants is discussed in terms of reduced endocytosis leading, concomitantly with aluminum tolerance, to changes in polar auxin transport.





Similar content being viewed by others
Abbreviations
- Al:
-
Aluminum
- AlRes:
-
Aluminum tolerance
- MS:
-
Murashige and Skoog medium
References
Ahad A, Wolf J, Nick P (2003) Isolation of tobacco mutants with increased tolerance to low temperature and anticytoskeletal herbicides by activation tagging. Trans Res 12:615–629
Bennet RJ, Breen CM (1991) The aluminum signal: new dimensions to mechanisms of aluminum tolerance. Plant Soil 134:153–166
Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273:948–950
Blancaflor EB, Jones DL, Gilroy S (1998) Alteration in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol 118:159–172
Breitling F, Little M (1986) Carboxy-terminal regions on the surface of tubulin and microtubules. Epitope Locations of YOL1/34, DM1A and DM1B. J Mol Biol 189:367–370
Chang C, Kwok SF, Bleecker AB, Myerowitz EM (1993) Arabidopsis ethylene/response gene ETR1: similarity of product to two-component regulators. Science 262:539–544
Chenery EM, Sporne KR (1976). A note on the evolutionary status of aluminum-accumulators among dicotyledons. New Phytol 76:551–554
Coué M, Brenner SL, Spector I, Korn ED (1987) Inhibition of actin polymerization by latrunculin A. FEBS Lett 213:316–318
De la Fuente JM, Ramirez-Rodriguez V, Carbera-Ponce JL, Herrera-Estrella L (1997) Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science 276:1566–1568
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107:315–321
Delhaize E, Hebb DM, Ryan PR (2001) Expression of a pseudomonas aeruginosa citrate synthase gene in tobacco is not associated with either enhanced citrate accumulation or efflux. Plant Physiol 125:2059–2067
Doncheva S, Amenós M, Poschenrieder C, Barceló J (2005) Root cell patterning: a primary target for aluminium toxicity in maize. J Exp Bot 56: 1213–1220
Foey CD, Chaney RL, White MS (1978) The physiology of metal toxicity in plants. Annu Rev Plant Physiol 29:511–566
Friml J (2003) Auxin transport—shaping the plant. Curr Opin Plant Biol 6:7–12
Frantzios G, Galatis B, Apostolakos P (2005) Aluminium causes variable responses in actin filament cytoskeleton of the root tip cells of Triticum turgidum. Protoplasma 225:129–140
Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly AI, Richards EL, Ejendal KFK, Smith AP, Baroux C, Grossniklaus U, Müller A, Hrycyna CA, Dudler R, Murphy AS, Martinoia E (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44:179–194
Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413:425–428
Geldner N, Richter S, Vieten A, Marquardt S, Torres-Ruiz RA, Mayer U, Jürgens G (2003) Partial loss of function alleses reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131:380–400
Godbolé R, Michalke W, Nick P, Hertel R (2000) Cytoskeletal drugs and gravity-induced lateral auxin transport in rice coleoptiles. Plant Biol 2:176–181
Grabski S, Schindler M (1995) Aluminum induces rigor within the actin network of soybean cells. Plant Physiol 108:897–901
Hasenstein KH, Evans ML (1988) Effects of cations on hormone transport in primary roots of Zea mays. Plant Physiol 86:890–894
Hayashi H, Czaja I, Schell J, Walden R (1992) Activation of plant gene by T-DNA tagging: Auxin independent growth in vitro. Science 258:1350–1353
Holweg C, Nick P (2004) Arabidopsis myosin XI mutant is defective in organelle movement and polar auxin transport. Proc Natl Acad Sci USA 101:10488–10493
Holweg C, Süßlin C, Nick P (2004) Capturing in-vivo dynamics of the actin cytoskeleton stimulated by auxin or light. Plant Cell Physiol 45:855–863
Kakimoto T (1996) CKI1, a histidine kinase homologue implicated in cytokinin signal transduction. Science 274:982–985
Kerven GL, Edwards DG, Asher CJ, Hallman PS, Kokot S (1989) Aluminum determination in soil solution. II. Short term calorimetric procedures for the measurement of inorganic monomeric aluminium in the presence of organic acid ligands. Austr J Soil Res 27:91–102
Kochian LV, Hoekenga OA, Piñeros MA (2005) How do crop plants tolerate acid soil? Mechanisms of aluminum tolerance and phosphorus efficiency. Annu Rev Plant Biol 55:459–493
Kollmeier M, Hubert HF, Horst JW (2000) Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol 122:945–956
Koncz C, Mayerhofer R, Konct-Kalman Z, Nawrath C, Reiss B, Redei GP, Schell J (1990) Isolation of a gene encoding a novel chloroplast protein by T-DNA tagging in Arabidopsis thaliana. EMBO J 9:1337–1346
Koncz C, Martini N, Szabados L, Hrouda M, Bachmair A, Schell J (1994) Specialized vectors for gene tagging and expression studies. In: Gelvin SB, Schilperoort RA, Verma DPS (eds) Plant molecular biology manual, vol B2. Kluwer Academic Publishers, Netherlands, pp 1–22
Koop H-U, Steinmüller K, Wagner H, Rössler C, Eibil C, Sacher L (1996) Integration of foreign sequences into the tobacco plastome via polyethylene glycol-mediated protoplast transformation. Planta 199:193–201
MacDonald TL, Martin RB (1988) Aluminum ion in biological systems. Trends Biochem Sci 13:15–19
Mattson J, Sung ZR, Berleth T (1999) Responses of plant vascular systems to auxin transport inhibition. Development 126:2979–2991
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakazawa M, Ichikawa T, Ishikawa A, Kobayashi H, Tsuhara Y, Kawashima M, Suzuki K, Muto S, Mastui M (2003) Activation tagging, a novel tool to dissect the functions of a gene family. Plant J 34:741–750
Nick P. (2006) Noise yields order: auxin, actin, and polar patterning. Plant Biol 8:360–370
Nick P, Lambert AM, Vantard M (1995) A microtubules-associated protein in maize is induced during phytochrome-dependent cell elongation. Plant J 8:835–844
Nick P, Heuing A, Ehmann B (2000) Plant chaperonins: a role in microtubule-dependent wall formation. Protoplasma 211:234–244
Popov N, Schmitt S, Matthices H (1975) Eine störungsfreie Mikromethode zur Bestimmung des Proteingehalts in Gewebshomogenaten. Acta Biol Ger 31:1441–1446
Piñeros MA, Shaff JE; Manslank HS, Alves VM, Kochian LV (2005) Aluminum resistance in maize cannot solely explained by root organic acid exudation. A comparative physiological study. Plant Physiol 137:231–241
Rengel Z, Zhang W-H (2003) Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytol 159:295–314
Ryan PR, Delhaize E, Randall PJ (1995) Malate efflux from root apices and tolerance to Al are highly correlated in wheat. Austr J Plant Physiol 22:531–536
Sachs T (2000) Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol 41:649–656
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum activated malate transporter. Plant J 37:645–653
Schmidt R, Bohm K, Vater W, Unger E (1991) Aluminum-induced osteomalacia and encelopathy: an aberration of the tubulin assembly into microtubules by Al3+. Prog Histochem Cytochem 23:355–364
Schwarzerová K, Zelenková S, Nick P, Opartrný Z (2002) Aluminum induced rapid changes in the microtubular cytoskeleton of tobacco cell lines. Plant Cell Physiol 43:207–216
Sivaguru M, Matsumoto H, Horst WJ (2000) Control of the response to aluminium stress. In: Nick P (ed) Plant microtubules—potential for biotechnology, Springer, Berlin Heidelberg New York , pp 103–120
Sivaguru M, Ezaki B, He Zh-H, Tong H, Osawa H, Baluška F, Volkmann D, Matsumoto H (2003a) Aluminum-induced gene expression and protein localization of a cell wall-associated receptor kinase in Arabidopsis. Plant Physiol 132:2256–2266
Sivaguru M, Pike S, Gassmann W, Baskin TI (2003b) Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol 44:667–675
Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Galweiler L, Palme K, Jurgens G (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286:316–318
Strzelecka-Golaszewska H (2001) Divalent cations, nucleotides, and actin structure. Res Probl Cell Differ 32:23–41
Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15:2648–2653
Tani H, Chen X, Nurmberg P, Grant JJ, SantaMaria M, Chini A, Gilroy E, Birch PRJ, Loake GJ (2004) Activation tagging in plants: a tool for gene discovery. Funct Integr Genomics 0:1–9
Vitorello VA, Haug A (1999) Capacity for aluminium uptake depends on brefeldin A-sensitive membrane traffic in tobacco (Nicotiana tabacum L. cv. BY-2) cells. Plant Cell Rep 18:733–736
Wagatsuma T, Kaneko M, Hayasaka Y (1995) Destruction process of plant root cells by aluminium. Soil Sci Plant Nutr 33:161–175
Waller F, Nick P (1997) Response of actin microfilaments during phytochrome-controlled growth of maize seedlings. Protoplasma 200:154–162
Waller F, Riemann M, Nick P (2002) A role for actin-driven secretion in auxin-induced growth. Protoplasma 219:72–81
Watt DA (2003) Aluminium-responsive genes in sugarcane: identification and analysis of expression under oxidative stress. J Exp Bot 54:1163–1174
Weigel D, Ahn JH, Blazquez MA et al (2000) Activation tagging in Arabidopsis. Plant Physiol 122:1003–1013
Xia Y, Suzuki H, Borevitz J, Blount J, Guo Z, Patel K, Dixon RA, Lamb C (2004) An extracellular aspartic protease functions in Arabidopsis disease resistance signaling. EMBO J 23:980–988
Yang JL, Zheng SJ, He YF, Matsumoto H (2005) Aluminum resistance requires resistance to acid stress: a case study with spinach that exudes oxlate rapidly when exposed to Al stress. J Exp Bot 56:1197–1203
Zubko E, Adams CJ, Machaelkova I, Malbeck J, Scollan C, Meyer P (2002) Activation tagging identifies a gene from Petunia hybrida responsible for the production of active cytokinins in plants. Plant J 29:797–808
Acknowledgments
This work was supported by a Volkswagen-Foundation Young Researcher Group Grant (Nachwuchsgruppe) to PN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahad, A., Nick, P. Actin is bundled in activation-tagged tobacco mutants that tolerate aluminum. Planta 225, 451–468 (2007). https://doi.org/10.1007/s00425-006-0359-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0359-0