Abstract
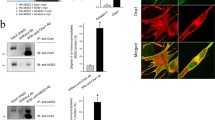
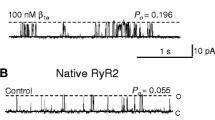
Skeletal muscle excitation–contraction (E–C) coupling is altered in several models of phosphatidylinositol phosphate (PtdInsP) phosphatase deficiency and ryanodine receptor activity measured in vitro was reported to be affected by certain PtdInsPs, thus prompting investigation of the physiological role of PtdInsPs in E–C coupling. We measured intracellular Ca2+ transients in voltage-clamped mouse muscle fibres microinjected with a solution containing a PtdInsP substrate (PtdIns(3,5)P 2 or PtdIns(3)P) or product (PtdIns(5)P or PtdIns) of the myotubularin phosphatase MTM1. No significant change was observed in the presence of either PtdIns(5)P or PtdIns but peak SR Ca2+ release was depressed by ~30% and 50% in fibres injected with PtdIns(3,5)P 2 and PtdIns(3)P, respectively, with no concurrent alteration in the membrane current signals associated with the DHPR function as well as in the voltage dependence of Ca2+ release inactivation. In permeabilized muscle fibres, the frequency of spontaneous Ca2+ release events was depressed in the presence of the three tested phosphorylated forms of PtdInsP with PtdIns(3,5)P 2 being the most effective, leading to an almost complete disappearance of Ca2+ release events. Results support the possibility that pathological accumulation of MTM1 substrates may acutely depress ryanodine receptor-mediated Ca2+ release. Overexpression of a mCherry-tagged form of MTM1 in muscle fibres revealed a striated pattern consistent with the triadic area. Ca2+ release remained although unaffected by MTM1 overexpression and was also unaffected by the PtdIns-3-kinase inhibitor LY2940002, suggesting that the 3-phosphorylated PtdIns lipids active on voltage-activated Ca2+ release are inherently maintained at a low level, inefficient on Ca2+ release in normal conditions.






Similar content being viewed by others
References
Al-Qusairi L, Weiss N, Toussaint A, Berbey C, Messaddeq N, Kretz C, Sanoudou D, Beggs AH, Allard B, Mandel JL, Laporte J, Jacquemond V, Buj-Bello A (2009) T-tubule disorganization and defective excitation-contraction coupling in muscle fibres lacking myotubularin lipid phosphatase. Proc Natl Acad Sci U S A 106:18763–18768
Amoasii L, Hnia K, Chicanne G, Brech A, Cowling BS, Müller MM, Schwab Y, Koebel P, Ferry A, Payrastre B, Laporte J (2013) Myotubularin and PtdIns3P remodel the sarcoplasmic reticulum in muscle in vivo. J Cell Sci 126:1806–1819
Bolis A, Zordan P, Coviello S, Bolino A (2007) Myotubularin-related (MTMR) phospholipid phosphatase proteins in the peripheral nervous system. Mol Neurobiol 35:308–316
Cao C, Backer JM, Laporte J, Bedrick EJ, Wandinger-Ness A (2008) Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol Biol Cell 19:3334–3346
Cheng H, Lederer WJ, Cannell MB (2003) Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science 262:740–744
Chu A, Stefani E (1991) Phosphatidylinositol 4,5-bisphosphate-induced Ca2+ release from skeletal muscle sarcoplasmic reticulum terminal cisternal membranes. Ca2+ flux and single channel studies. J Biol Chem 266:7699–7705
Clark J, Anderson KE, Juvin V, Smith TS, Karpe F, Wakelam MJ, Stephens LR, Hawkins PT (2011) Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nat Methods 8:267–272
Collet C, Csernoch L, Jacquemond V (2003) Intramembrane charge movement and L-type calcium current in skeletal muscle fibres isolated from control and mdx mice. Biophys J 84:251–265
Collet C, Pouvreau S, Csernoch L, Allard B, Jacquemond V (2004) Calcium signaling in isolated skeletal muscle fibres investigated under "Silicone Voltage-Clamp" conditions. Cell Biochem Biophys 40:225–236
Csernoch L, Bernengo JC, Szentesi P, Jacquemond V (1998) Measurements of intracellular Mg2+ concentration in mouse skeletal muscle fibres with the fluorescent indicator mag-indo-1. Biophys J 75:957–967
Csernoch L, Pouvreau S, Ronjat M, Jacquemond V (2008) Voltage-activated elementary calcium release events in isolated mouse skeletal muscle fibres. J Membr Biol 226:43–55
Di Paolo G, De Camilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443:651–657
Dowling JJ, Low SE, Busta AS, Feldman EL (2010) Zebrafish MTMR14 is required for excitation–contraction coupling, developmental motor function and the regulation of autophagy. Hum Mol Genet 19:2668–2681
Dowling JJ, Vreede AP, Low SE, Gibbs EM, Kuwada JY, Bonnemann CG, Feldman EL (2009) Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet 5:e1000372. doi:10.1371/journal.pgen.1000372
Eisenberg BR (1983) Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, Adrian RH, Geiger SR (eds) Handbook of physiology sect 10: skeletal muscle, 3rd edn. Williams & Wilkins, Baltimore, MD, pp 73–112
Hannon JD, Lee NK, Yandong C, Blinks JR (1992) Inositol trisphosphate (InsP3) causes contraction in skeletal muscle only under artificial conditions: evidence that Ca2+ release can result from depolarization of T-tubules. J Muscle Res Cell Motil 13:447–456
Harkins AB, Kurebayashi N, Baylor SM (1993) Resting myoplasmic free calcium in frog skeletal muscle fibres estimated with fluo-3. Biophys J 65:865–881
Jacquemond V (1997) Indo-1 fluorescence signals elicited by membrane depolarization in enzymatically isolated mouse skeletal muscle fibres. Biophys J 73:920–928
Kirsch WG, Uttenweiler D, Fink RH (2001) Spark- and ember-like elementary Ca2+ release events in skinned fibres of adult mammalian skeletal muscle. J Physiol 537:379–389
Kobayashi M, Muroyama A, Ohizumi Y (1989) Phosphatidylinositol 4,5-bisphosphate enhances calcium release from sarcoplasmic reticulum of skeletal muscle. Biochem Biophys Res Commun 163:1487–1491
Kong D, Dan S, Yamazaki K, Yamori T (2010) Inhibition profiles of phosphatidylinositol 3-kinase inhibitors against PI3K superfamily and human cancer cell line panel JFCR39. Eur J Cancer 46:1111–1121
Lanner JT, Georgiou DK, Joshi AD, Hamilton SL (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2:a003996
Laporte J, Hu LJ, Kretz C, Mandel JL, Kioschis P, Coy JF, Klauck SM, Poustka A, Dahl N (1996) A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet 13:175–182
Lemmon MA (2008) Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Bio 9:99–111
Logothetis DE, Petrou VI, Adney SK, Mahajan R (2010) Channelopathies linked to plasma membrane phosphoinositides. Pflugers Arch 460:321–341
Lukács B, Sztretye M, Almássy J, Sárközi S, Dienes B, Mabrouk K, Simut C, Szabó L, Szentesi P, De Waard M, Ronjat M, Jóna I, Csernoch L (2008) Charged surface area of maurocalcine determines its interaction with the skeletal ryanodine receptor. Biophys J 95:3497–3509
Monnier N, Ferreiro A, Marty I, Labarre-Vila A, Mezin P, Lunardi J (2003) A homozygous splicing mutation causing a depletion of skeletal muscle RYR1 is associated with multi-minicore disease congenital myopathy with ophthalmoplegia. Hum Mol Genet 12:1171–1178
Ogawa Y, Harafuji H (1989) Ca-release by phosphoinositides from sarcoplasmic reticulum of frog skeletal muscle. J Biochem 106:864–867
Ohizumi Y, Hirata Y, Suzuki A, Kobayashi M (1999) Two novel types of calcium release from skeletal sarcoplasmic reticulum by phosphatidylinositol 4,5-biphosphate. Can J Physiol Pharmacol 77:276–285
Pierson CR, Tomczak K, Agrawal P, Moghadaszadeh B, Beggs AH (2005) X-linked myotubular and centronuclear myopathies. J Neuropathol Exp Neurol 64:555–564
Pouvreau S, Csernoch L, Allard B, Sabatier JM, De Waard M, Ronjat M, Jacquemond V (2006) Transient loss of voltage control of Ca2+ release in the presence of maurocalcine in skeletal muscle. Biophys J 91:2206–2215
Pouvreau S, Jacquemond V (2005) Nitric oxide synthase inhibition affects sarcoplasmic reticulum Ca2+ release in skeletal muscle fibres from mouse. J Physiol 567:815–728
Romero-Suarez S, Shen J, Brotto L, Hall T, Mo C, Valdivia HH, Andresen J, Wacker M, Nosek TM, Qu CK, Brotto M (2010) Muscle-specific inositide phosphatase (MIP/MTMR14) is reduced with age and its loss accelerates skeletal muscle aging process by altering calcium homeostasis. Aging 2:504–513
Shen J, Yu WM, Brotto M, Scherman JA, Guo C, Stoddard C, Nosek TM, Valdivia HH, Qu CK (2009) Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca2+ homeostasis. Nat Cell Biol 11:769–776
Suh BC, Hille B (2008) PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37:175–195
Szabó LZ, Vincze J, Csernoch L, Szentesi P (2010) Improved spark and ember detection using stationary wavelet transforms. J Theor Biol 264:1279–1292
Zhou H, Jungbluth H, Sewry CA, Feng L, Bertini E, Bushby K, Straub V, Roper H, Rose MR, Brockington M, Kinali M, Manzur A, Robb S, Appleton R, Messina S, D'Amico A, Quinlivan R, Swash M, Müller CR, Brown S, Treves S, Muntoni F (2007) Molecular mechanisms and phenotypic variation in RYR1-related congenital myopathies. Brain 130:2024–2036
Zhou H, Lillis S, Loy RE, Ghassemi F, Rose MR, Norwood F, Mills K, Al-Sarraj S, Lane RJ, Feng L, Matthews E, Sewry CA, Abbs S, Buk S, Hanna M, Treves S, Dirksen RT, Meissner G, Muntoni F, Jungbluth H (2010) Multi-minicore disease and atypical periodic paralysis associated with novel mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord 20:166–173
Zhou H, Yamaguchi N, Xu L, Wang Y, Sewry C, Jungbluth H, Zorzato F, Bertini E, Muntoni F, Meissner G, Treves S (2006) Characterization of recessive RYR1 mutations in core myopathies. Hum Mol Genet 15:2791–2803
Acknowledgements
This work was supported by grants from Centre National de la Recherche Scientifique (CNRS), Université Lyon 1, Association Française contre les Myopathies (AFM), Hubert Curien Partnership Balaton (TeT_10-1-2011-0723) and OTKA K107765. E.G.R. was a recipient of a fellowship from the Spanish Ministry of Education and Science (MEC, José Castillejo Program). We thank Bruno Allard for critical comments on the manuscript and helpful discussion.
Ethical standards
All experiments comply with the current laws of the two countries (France and Hungary) in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 544 kb)
Rights and permissions
About this article
Cite this article
González Rodríguez, E., Lefebvre, R., Bodnár, D. et al. Phosphoinositide substrates of myotubularin affect voltage-activated Ca2+ release in skeletal muscle. Pflugers Arch - Eur J Physiol 466, 973–985 (2014). https://doi.org/10.1007/s00424-013-1346-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1346-5