Abstract
Objective
MATR3-associated distal myopathy is a rare distal myopathy predominantly affecting lower legs as well as wrist- and finger extensors. Whilst most distal myopathies are clinically and genetically well characterized, diagnosis often remains challenging. Pattern-based magnetic resonance imaging (MRI) approaches offer valuable additional information. However, a consistent pattern of muscular affection is missing for most distal myopathies. Thus, the aim of the present study was to establish a disease-specific pattern of muscular involvement in MATR3-associated distal myopathy using whole-body MRI.
Methods
15 patients (25–79 years of age, 7 female) with MATR3-associated distal myopathy were subjected to whole-body MRI. The grade of fatty involution for individual muscles was determined using Fischer-Grading. Results were compared to established MRI-patterns of other distal myopathies.
Results
There was a predominant affection of the distal lower extremities. Lower legs showed a severe fatty infiltration, prominently affecting gastrocnemius and soleus muscle. In thighs, a preferential involvement of semimembranous and biceps femoris muscle was observed. Severe affection of gluteus minimus muscle as well as axial musculature, mainly affecting the thoracic segments, was seen. A sufficient discrimination to other forms of distal myopathy based solely on MRI-findings of the lower extremities was not possible. However, the inclusion of additional body parts seemed to yield specificity.
Interpretation
Muscle MRI of patients with MATR3-associated distal myopathy revealed a distinct pattern of muscular involvement. The usage of whole-body muscle MRI provided valuable additional findings as compared to regular MRI of the lower extremities to improve distinction from other disease entities.
Similar content being viewed by others
Introduction
Distal myopathies are a heterogeneous group of rare muscular disorders that vary in disease onset, mode of inheritance and clinical presentation [1]. Recent advances in genetics have broadened the genotypic spectrum of distal myopathies enormously. To date, nearly 20 distinct disease entities have been identified as classical distal myopathies. Moreover, a variety of muscular disorders show a facultative distal phenotype. Thus, the identification of the correct genotype as well as the evaluation of genetic variants of uncertain significance remain challenging and require a thorough clinical workup [2].
Imaging of the musculature offers valuable additional information to facilitate the diagnostic process as specific patterns of muscular involvement—with some muscles being selectively affected whilst others are spared—have been observed in different muscle diseases [3]. There are various studies that have aimed to establish specific patterns of muscular affection within different distal myopathies (e.g. [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27].). However, the majority of reports rely on a relatively small number of individuals studied. Furthermore, most studies are lacking a systematic quantitative and qualitative assessment of the individual muscles (by means of a grading system) as well as information regarding inter-individual variability. In addition, selective imaging of the lower extremities instead of whole-body examination was used for pattern definition in most cases. Consistently, pattern-based approaches to identify the underlying genotype of patients with distal myopathy have yielded low sensitivity and specificity [28]. Hence, a structured evaluation of preferably large cohorts is needed to refine the individual patterns of muscular involvement and to improve the predictive value of information gained by muscle imaging.
Matrin-3-associated distal myopathy is a rare late-onset distal myopathy predominantly affecting the lower legs as well as finger and wrist extensors [29]. Dysphagia, dysphonia and a restrictive ventilation disorder are observed frequently throughout disease progression [30]. Since its first description as Vocal cord and pharyngeal weakness with distal myopathy (VCPDM) in 1998 and subsequent identification of the causative mutation in the MATR3-Gene (p.S85C, c.254C > G), a number of patients with Matrin-3-associatied distal myopathy have been identified. Hence, the clinical phenotype has been profoundly characterized [29, 31,32,33,34,35]. Although some of these reports also included muscle imaging, a comprehensive evaluation in terms of specific patterns, frequency of occurrence and severity of the observed muscular affection as well as dynamics throughout disease progression is missing [29, 31, 33, 35].
In this study, whole body-MRI scans of patients with genetically proven Matrin-3-associated distal myopathy were used with the aim to identify a specific pattern of muscular affection and to monitor the sequence of involvement. Furthermore, the obtained pattern was compared to findings in other distal myopathies to address the diagnostic value of the identified changes.
Patients and methods
Patients
Patients with genetically confirmed Matrin-3-associated distal myopathy (p.S85C/c.254C > G mutation) who underwent whole-body MRI in the course of routine diagnostic work-up were asked for permission to re-evaluate the obtained data for the present study. 15 patients (25–79 years of age, 7 female) out of 8 families were included in the current study. The majority of the patients (9 patients) were in mid-stage of the disease (41–55 years of age, disease duration 5–29 years), whilst each three were classified to be in disease onset (25–30 years of age, presymptomatic or myalgia only) and end-stage (67–79 years of age, disease duration 36–39 years, disease duration not known in one case) disease, respectively. While 4 of the patients (patients 4, 5, 10, 11) were previously described [29], 4 newly identified unrelated pedigrees were included in the current study. Table 1 summarizes the main characteristics of the patients.
MRI protocol and evaluation
Whole-body-Imaging was done on a 3.0 T MRI-system (Skyra; Siemens, Erlangen, Germany) using two flexible 18-channel transmit/receive surface body coils for neck, thorax, arms, abdomen and pelvis. A 36-channel angiography coil was used for the legs. The patient was placed in the supine position. Imaging included a T1-Weighted Dixon Turbo Spin Echo sequence in an axial plain and 8 mm slice thickness. The total amount of time required for the MRI examination was 45 min. No sedation or contrast agent was used.
The grade of affection of the individual muscle was determined using the five-point semi-quantitative grading scale established by Fischer et al. [10]. Briefly, stage 0 refers to a normal appearance, while decreased T1-signal intensity to a variable extent is found in stages 1–4 (1—slight, non-confluencing; 2—beginning confluence in less than 50% of the muscle; 3—confluence in more than 50% of the muscle; 4—replacement of the entire muscle).
Determination was conducted by an experienced radiologist (DS) who was blinded to the clinical data. A complete summary showing the individual patients’ determined Fischer-grades can be found in Suppl. Table 1.
MATR3 MRI-pattern definition
To establish a comprehensive MRI-pattern of muscular affection, patients were first stratified in regard to disease duration (disease onset, mid-stage and end-stage disease). For pattern definition, only advanced disease stages (e.g. mid- and end-stage) were used. For each individual muscle, average as well as median Fischer grade and standard deviation was determined. Using these parameters, the grade of affection was classified into four categories—not/rarely affected (average Fischer grade 0–1.4, median Fischer grade 0), mild/variable affected (average Fischer grade 1.5–2.4, median Fischer grade 1–2, standard deviation > 1.5), moderately affected (average Fischer grade 2.5–3.4, median Fischer grade 3) and early/severely affected (average Fischer grade ≥ 3.5, median Fischer grade 4).
Pattern evaluation
To test the reliability of the obtained MRI-pattern, all patients with MATR3-myopathy included in this study were compared to the MRI-pattern of muscular affection identified in this study. The derived MATR3 MRI-pattern was dichotomized, only distinguishing between unaffected (not or mild/variable affected) and affected (moderately and early/severely affected) muscles. In patients, all muscles with a Fischer grade > 1 were considered to be affected. For each patient, the mismatch to the defined MATR3-pattern was determined. In this regard, a mismatch was defined as either a muscle that was affected in the individual patient but not in the defined MATR3-pattern or vice versa a muscle that appeared to be not affected in the individual patient but was defined affected in the MATR3-pattern. A mismatch ≤ 20% of all muscles studied in the individual patient was supposed a good congruency to the MATR3-pattern. Hence, the portion of patients fulfilling this criterion was identified.
Comparison of MATR3 MRI-pattern to other distal myopathies
Patterns of muscular involvement for the individual distal myopathies were defined on the basis of previous findings in distal myopathies. The work of Bugiardini et al. served as the basis for pattern definition [28]. Furthermore, a selective literature review was done using the terms “MRI”, “magnetic resonance imaging”, “MR” and “imaging” in connection with “distal myopathy” as well as the respective disease entities. A comprehensive list of the literature used for pattern definition can be found in Suppl. Table 2. In addition to the known “classical” distal myopathies (obligatory distal phenotype), several myopathies with comparably high prevalence and potential predominant distal affection were included (Facioscapulohumeral muscular dystrophy 1, Myotonic dystrophy 1, sporadic Inclusion body myositis, Hereditary myopathy with early respiratory failure).
The established literature-based pattern was then compared to MATR3-pattern and mismatch was determined. Again, a mismatch ≤ 20% was defined as relevant congruency.
Results
Muscular affection in MATR3-myopathy
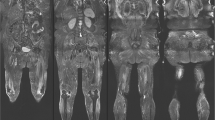
Muscular involvement was mainly symmetrical. A predominant affection of the lower extremities with an emphasis on the lower legs was observed. In lower legs, a predominant involution of soleus muscle as well as medial head of gastrocnemius muscle was detected. However, the anterior and lateral compartment (e.g. tibialis anterior, extensor digitorum longus and peroneus longus muscle) were also involved in most of the cases, though to a lesser extent. A variable degeneration of lateral head of gastrocnemius muscle was seen. However, the deep posterior compartment was mainly unaffected. In thighs, a selective affection of parts of the hamstring muscles (semimembranosus muscle and the long head of the biceps femoris muscle) was observed whereas the anterior and medial compartments were mostly spared. Imaging of the trunk showed early and severe involvement of the gluteus minimus muscle. In contrast, gluteus medius muscle showed a variable grade of affection while gluteus maximus muscle appeared to be not relevantly involved. Evaluation of the paraspinal musculature revealed a scattered degeneration predominantly affecting thoracic parts of the axial musculature (e.g. thoracis longus, semispinalis thoracis and spinalis thoracis muscles). The cervical and lumbar parts of paraspinal musculature appeared only mildly affected. Except for the deltoid muscle, that—especially in the ventral part—showed a moderate involution, no relevant involvement of the musculature of shoulder and arm was seen. Representative examples of whole body imaging findings in MATR3-myopathy are depicted in Fig. 1 while the observed grade of alteration for the individual muscles as determined by Fischer grading are shown in Fig. 2.
Magnetic resonance imaging in MATR3-associated distal myopathy. Representative T1-weighted MRI slices of head/neck, shoulder/thorax, trunk, pelvis, upper and lower leg in patients with different disease stages of MATR3-associated distal myopathy. Note the early affection of gluteus minimus muscle (white arrowhead) and thoracic segments of paraspinal musculature (dashed arrowhead) while gluteus medius and maximus muscles as well as cervical and lumbar segments of paraspinal musculature are relatively spared. DD disease duration, y years, F female, M male
Grade of affection for individual muscles as determined by Fischer grading. The proportion of respective Fischer grades (Fisher 1–Fischer 4) observed in individual muscles is shown for all patients (a) as well as disease onset, mid- and end-stage disease (b). DD disease duration, y years, Spl. Splenius, maj. major, min. minor, long. longus, thor. thoracis, dors. dorsi, ant. anterior, prof. profundus, sup. superficialis, dig. digitorum, abd. abdominis, int. internus, ext. externus, max. maximus, med. medius, min. minumus, brev. brevis, magn. magnus, fasc. lat. fasciae latae, fem. femoris, med. medialis, intermed. intermedius, lat. lateralis, cap. brev. caput breve, cap. long. caput longum, cap. med. caput mediale, cap. lat. caput laterale
Dynamics throughout disease progression
At disease onset, there was already a slight (Fischer Grade 1 and 2) involvement in muscles that appeared to be severely affected in advanced disease stages. Especially, gluteus medius muscle as well as the lower limb muscles (e.g. soleus and peroneus longus muscles and the medial head of the gastrocnemius) showed mild involution in some of the patients (Fig. 2b, upper panel). During the course of the disease, a contemporaneous degeneration of the typically affected muscles was observed (Fig. 2b, middle panel), while not or rarely affected muscles appeared to be spared up to late stages of the disease, showing only mild involution that could be ascribed to age-related changes (Fig. 2b, lower panel).
Pattern definition and evaluation
A comprehensive pattern of muscular involvement in MATR3-associated myopathy was defined on the basis of the Fischer grading system (for details see “Methods” section). Three muscles, namely soleus muscles, the medial head of gastrocnemius muscle and gluteus minimus muscle were identified as early/severely affected. Tibialis anterior, extensor digitorum, peroneus longus and semimembranosus muscles as well as the thoracic parts of paraspinal musculature were categorized as being moderately affected. A mild/variable involution was identified for the lateral head of gastrocnemius and biceps femoris muscles, gluteus medius muscle, deltoideus muscle and cervical/lumbar parts of paraspinal musculature. All other muscles were defined as been not or rarely affected. A comprehensive summary of the defined MATR3-pattern is given in Fig. 3a.
Established pattern of muscular affection in MATR3-associated distal myopaty. a Schematic representation of the established pattern of muscular affection in MATR3-associated distal myopathy, stratified to not/rarely, mild/variable, moderate and early/severely affected. b Evaluation of the obtained pattern in patients with MATR3-myopathy as shown by match/mismatch of the individual muscles to the established pattern. Mass masseter, SCM sternocleidomastoid, Tr trapezius, Spc splenius capitis, Spcer splenius cervicis, SSc/cer semispinalis capitis/cervicis, LS levator scapulae, D deltoideus, Pmaj pectoralis major, Pmin pectoralis minor, SSp supraspinatus, IS infraspinatus, Tmi teres minor, SuS subscapularis, TL thoracic longissimus, SsTh semispinalis thoracis, STh spinalis thoracis, RH rhomboideus major, LD latissimus dorsi, SA serratus anterior, IC intercostal, BB biceps brachii, Br brachialis, TB triceps brachii, CoBr coracobrachialis, Fdsu flexor digitorum superficialis, FDpr flexor digitorum profundus, Edm extensor digitorum manus, RA rectus abdominis, OE obliquus externus, OI obliquus internus, TR transversus abdominis, IL iliacus, PS psoas, Mu multifidus, SaS sacrospinalis, GMax gluteus maximus, GMed gluteus medius, GMin gluteus minimus, AL adductor longum, AM adductor magnus, RF rectus femoris, VM vastus medialis, VI vastus intermedius, VL vastus lateralis, G gracilis, S sartorius, ST semitendinosus, SM semimembranosus, BF c.br. biceps femoris short head (caput breve), BF c.l. biceps femoris long head (caput longum), TA tibialis anterior, ED extensor digitorum, P popliteus, TP tibialis posterior, SO soleus, GCM gastrocnemius medialis, GCL gastrocnemius lateralis, aff. affected
To evaluate the practicability of the defined MATR3-pattern, mismatches to the findings in the individual patients were identified (Fig. 3b, Table 2). For all patients, a median mismatch of 6 muscles (12% of all examined muscles) was determined. The best match to the defined pattern was observed in the group of individuals with mid-stage disease, showing a median mismatch of 2 muscles (4%). While patients in end-stage disease showed a rather good match to the defined MATR3 pattern (median mismatch 7 muscles), a consistently poor match was seen in the disease onset group (median mismatch 10 muscles).
Overall, in 11 out of 15 patients (73%) a mismatch less than 20% of all examined muscles was observed. None of the patients in the disease onset group showed a sufficient match with the MATR3-pattern. A sufficient congruency (defined as mismatch < 20%) was observed for 89% of the patients in mid-stage and 100% of the patients in end-stage disease. An even stricter match to the defined MATR3-pattern (mismatch < 10%) was observed in 5 out of 9 patients in mid-stage disease (55%), while only one specimen (33%) in late-stage disease fulfilled this criterion.
Comparison to other distal myopathies
To determine the diagnostic practicability of the established MATR3-pattern, it was compared to literature-based lower-limb MRI patterns of other distal myopathies (Fig. 4a). A relevant discrimination (e.g. mismatch > 20%) of the MATR3-pattern to the majority of distal myopathies (16 out of 22 disease entities) was seen. In contrast, relevant similarities were observed in 6 distal myopathies (TIA1, late-stage TTN, MYOT, SQSTM1/TIA1, KLHL9, ADSSL1). Comprehensive information regarding affected muscles other than lower limb muscles were lacking for most of these distal myopathies, as only a few underlying studies applied whole-body MRI (2 out of 15, Table 3). Based on the available literature, a frequent affection of gluteal muscles was observed in MYOT- associated distal myopathy. However, a selective involution of gluteus minimus muscle as in MATR3-associated myopathy was not described (Table 3). Muscular involution of the paraspinal musculature was again solely reported in MYOT-associated distal myopathy. While a selective involvement of the thoracic paraspinal segments has not been described, there has been no explicit discrimination between lumbar, thoracic and cervical segments in this study (Table 3). Furthermore, respective changes were not present in MR-studies from patients with MYOT- and SQSTM1/TIA1-associated distal myopathy overseen in the neuromuscular research centre in Halle (Fig. 4b).
Comparison of the established MATR3-pattern to other distal myopathies. a Literature-based pattern of different distal myopathies and the respective mismatch to the established MATR3-pattern. Both ‘classical’ distal myopathies (obligatory distal phenotype), as well as myopathies with comparably high prevalence and potential predominant distal phenotype (potential distal PT) are included. Disease entities showing relevant similarities (mismatch < 20%) are highlighted in red. b Sample MRI findings in patients with distal myopathies determined to show similar patterns in lower limb muscular affection compared to MATR3-myopathy. Gluteal and paraspinal musculature is spared in patients with MYOT- and SQSTM1/TIA1-associated distal myopathy. DD disease duration, y years, PT phenotype, F female, M male, FSHD Facioscapulohumeral muscular dystrophy 1, DM1 Myotonic dystrophy 1, sIBM sporadic Inclusion body myositis, HMERF Hereditary myopathy with early respiratory failure, AL adductor longum, AM adductor magnus, RF rectus femoris, VM vastus medialis, VI vastus intermedius, VL vastus lateralis, G gracilis, S sartorius, ST semitendinosus, SM semimembranosus, BF c.br. biceps femoris short head (caput breve), BF c.l. biceps femoris long head (caput longum), TA tibialis anterior, ED extensor digitorum, P popliteus, TP tibialis posterior, SO soleus, GCM gastrocnemius medialis, GCL gastrocnemius lateralis, aff. affected
Discussion
In this study, a comprehensive pattern of muscular involvement in MATR3-associated distal myopathy has been established. To our knowledge, this is the largest cohort of patients with MATR3-myopathy studied so far. Furthermore, whole-body MRI as well as a semi-quantitative, scoring-based evaluation system is used for the first time to evaluate muscular affection in MATR3-myopathy.
Overall, the observed muscular involution meets very well with the typical clinical aspects seen in MATR3-myopathy (e.g. reported in [29, 32]). Corresponding to the initial symptoms complaint by the patients, a predominant affection of the distal lower limbs was seen. However, in contrast to the initially present bilateral foot-drop, MRI-evaluation showed the posterior compartment to be involved in early disease stages. One explanation of this discrepancy might be the larger volume of the posterior compartment in comparison to the anterior compartment and thus higher capacity to compensate for the beginning involution. Furthermore, most of the patients clinically exhibit a relevant weakness of wrist-/finger extensors, whereas respective MRI-changes were not seen in the present study. The low local resolution as well as the forearms being placed at the edge of the field of view and thus being particularly susceptible to artefacts may account for this phenomenon. Additional sequences focussing on the distal upper limbs or thin, flat muscles like trapezius muscle (e.g. coronal and sagittal sections) may further yield the diagnostic value but have to be carefully pondered against the increasing acquisition time (45 min with the protocol used in this study). The observed selective involvement of the thoracic segments of paraspinal musculature corresponds very well to the camptocormia frequently observed in advanced disease stages of MATR3 myopathy (unpublished observation).
Based on the grading system applied, a comprehensive pattern of muscular affection in MATR3-associated myopathy could be established, that showed a feasible match to the individual patients. However, validation of the MATR3-pattern was achieved solely on the basis of the patients that also served for pattern definition. This obvious limitation of the study mainly relates to the low prevalence of the disease and thus lack of additional individuals for thorough validation. In this regard, the only available publication addressing whole-body MRI in MATR3-associated distal myopathy apart from the present study is a case report describing one french patient [31]. The pattern of muscular involvement reported by Barp et al. fits well with the changes observed in the present study, although explicit information regarding individual muscle involvement is missing. Nonetheless, additional prospective studies including newly identified individuals with MATR3-myopathy are needed to further address this issue.
Overall, there was a good congruency of the MATR3-pattern with the individual muscular affection observed in patients. However, some patients (in particular patient 10 and 11) showed a relevant mismatch. Patient 10 had been subjected to high doses of glucocorticoids (non-related to muscular disease) for several years that might have led to additional muscular involution by means of a corticosteroid-induced myopathy. The discrepancies observed in this patient mainly apply to the proximal muscles of the lower extremity, which are predominantly affected in corticosteroid-induced myopathy [36, 37]. In Patient 11, the mismatch might at least in parts rely on excessive overweight (body mass index 44 kg/m2) leading to diffuse fatty infiltration of the musculature [38, 39]. In this regard, quantitative MRI assessment methods would be more feasible allowing normalisation to body mass index or mean fat fraction, respectively.
Noteworthy, MATR3-patients at disease onset (presymptomatic or myalgia only) did not match with the MATR3-pattern. Thus, the established pattern may only be useful in patients that show clinical weakness and not be suitable for early identification of oligosymptomatic patients. However, even patients that were imaged in a comparably short interval to symptom onset (patients 4 and 5, disease duration 5 and 8 years, respectively) displayed a good congruency to the defined pattern (mismatch 2% each). This suggests that the predominantly affected muscles are consistently involved at an early timepoint in disease progression.
When comparing the MATR3-pattern to literature-based patterns of other distal myopathies, a good distinction to most of the disease entities was seen. However, a relevant number of studies on disease-specific patterns were only based on a small sample size or did not apply semi-quantitative scoring systems. Furthermore, in most cases an explicit annotation regarding the individual grade of affection of all studied muscles is missing. Thus, the literature-based patterns used in this study might not fully account for the alterations to be expected in some disease entities. In this context, the missing validation of the defined patterns beyond literature evidence appears to be another limitation of the approach used in this study.
Interestingly, none of the myopathies with potential distal phenotype showed a relevant congruency to MATR3-myopathy. This might partly be due to the relevant affection of proximal muscle groups reported in all available MRI-studies concerning these disease entities. Reports addressing MRI-finding in patients with exclusive predominant distal affection are missing (see Suppl. Table 2 for details).
Despite the satisfying delineation from most distal myopathies using the findings of the lower limb alone, a relevant similarity was found for six distal myopathies. Hence, a sufficient discrimination on the basis of a sole examination of the lower limbs might not be possible.
Studies using whole-body MRI are missing for most of the distal myopathies with relevant congruency to MATR3-associated myopathy. On the basis of the available literature, patterns of gluteal and paraspinal affection found in MYOT-associated distal myopathy were divergent to the findings in MATR3-myopathy. This included also a patient with MYOT-associated distal myopathy overseen in the neuromuscular research centre in Halle. Furthermore, the only available study using whole-body MRI in MYOT-myopathy has reported frequent involvement of rhomboidei muscles as well as variable affection of proximal muscles of the upper extremities [25]. These findings were not observed in MATR3-myopathy and thus may further facilitate discrimination of both myopathies. Additionally, gluteal and paraspinal changes as observed in MATR3-myopathy were not seen in whole-body examination of a patient with SQSTM1/TIA1-associated distal myopathy, a distal myopathy that displays similar lower limb findings as compared to MATR3-associated myopathy.
Under this aspect, the usage of whole-body imaging offers additional information regarding sufficient delineation of different disease entities. Thus, the findings of the present study further support the recently published recommendations of the MYO-MRI consortium, emphasizing the role of whole-body MRI in diagnosing and monitoring neuromuscular disorders [40]. Whether the defined categories of muscular involvement, the used parameters and set margins are applicable for other studies using different disease entities remains elusive.
The approach presented in this study appears to be considerably operational. Integrational use of—among others—clinical presentation, family history and muscle biopsy findings along with MRI-examination should further facilitate the accuracy of discrimination between different distal myopathies. For instance, KLHL9- and ADSSL1-associated distal myopathies are reported to have a rather early onset between 10 and 20 years of age, while first symptoms in MYOT-associated distal myopathy are occurring between 50 and 60 years of age [7, 21, 22, 41, 42]. Thus, they might be easily distinguished from MATR3-myopathy that usually shows an onset between 30 and 40 years of age [29]. MYOT-myopathy usually presents with a myofibrillar histopathology, that has not been identified in MATR3-associated distal myopathy [43]. While TIA1- and SQSTM1/TIA1-associated distal myopathy display a rather identical clinical phenotype compared to MATR3 (in terms of onset, muscle weakness and histopathology), none of these disease entities typically include a relevant dysphagia and dysphonia [12, 16, 44, 45]. Autosomal dominant TTN-associated myopathy (Udd myopathy) shows no upper limb involvement and is usually restricted to the anterior compartment of the lower legs, while the posterior compartment is only mildly affected in late disease stages [14, 46]. Thus, a sufficient delineation to MATR3-associated myopathy appears feasible.
In summary, the distinct pattern of muscular affection established in this study expands the diagnostic opportunities in MATR3-associated distal myopathy in terms of discrimination to other distal myopathies. It emphasizes the benefits of semi-quantitative, scoring-based approaches and explicit delineation of all studied muscles. Furthermore, the data presented highlights the potential of whole-body examination for further delineation of different disease entities. In this respect, the conclusions drawn from this study do not only apply for distal myopathies but for MRI-based studies in neuromuscular disorders in general.
Availability of data and material
All data used for this study is available in deidentified form in the Electronic Supplementary Material section.
References
Udd B (2014) Distal myopathies. Curr Neurol Neurosci Rep 14(3):434. https://doi.org/10.1007/s11910-013-0434-4
Udd B (2012) Distal myopathies–new genetic entities expand diagnostic challenge. Neuromuscul Disord 22(1):5–12. https://doi.org/10.1016/j.nmd.2011.10.003
Leung DG (2017) Magnetic resonance imaging patterns of muscle involvement in genetic muscle diseases: a systematic review. J Neurol 264(7):1320–1333. https://doi.org/10.1007/s00415-016-8350-6
Ahlberg G, Jakobsson F, Fransson A, Moritz A, Borg K, Edstrom L (1994) Distribution of muscle degeneration in Welander distal myopathy–a magnetic resonance imaging and muscle biopsy study. Neuromuscul Disord 4(1):55–62. https://doi.org/10.1016/0960-8966(94)90048-5
Berciano J, Gallardo E, Dominguez-Perles R, Gallardo E, Garcia A, Garcia-Barredo R, Combarros O, Infante J, Illa I (2008) Autosomal-dominant distal myopathy with a myotilin S55F mutation: sorting out the phenotype. J Neurol Neurosurg Psychiatr 79(2):205–208. https://doi.org/10.1136/jnnp.2007.125435
Bucelli RC, Arhzaouy K, Pestronk A, Pittman SK, Rojas L, Sue CM, Evila A, Hackman P, Udd B, Harms MB, Weihl CC (2015) SQSTM1 splice site mutation in distal myopathy with rimmed vacuoles. Neurology 85(8):665–674. https://doi.org/10.1212/WNL.0000000000001864
Cirak S, von Deimling F, Sachdev S, Errington WJ, Herrmann R, Bonnemann C, Brockmann K, Hinderlich S, Lindner TH, Steinbrecher A, Hoffmann K, Prive GG, Hannink M, Nurnberg P, Voit T (2010) Kelch-like homologue 9 mutation is associated with an early onset autosomal dominant distal myopathy. Brain 133(Pt 7):2123–2135. https://doi.org/10.1093/brain/awq108
Diaz-Manera J, Fernandez-Torron R, LLauger J, James MK, Mayhew A, Smith FE, Moore UR, Blamire AM, Carlier PG, Rufibach L, Mittal P, Eagle M, Jacobs M, Hodgson T, Wallace D, Ward L, Smith M, Stramare R, Rampado A, Sato N, Tamaru T, Harwick B, Rico Gala S, Turk S, Coppenrath EM, Foster G, Bendahan D, Le Fur Y, Fricke ST, Otero H, Foster SL, Peduto A, Sawyer AM, Hilsden H, Lochmuller H, Grieben U, Spuler S, Tesi Rocha C, Day JW, Jones KJ, Bharucha-Goebel DX, Salort-Campana E, Harms M, Pestronk A, Krause S, Schreiber-Katz O, Walter MC, Paradas C, Hogrel JY, Stojkovic T, Takeda S, Mori-Yoshimura M, Bravver E, Sparks S, Bello L, Semplicini C, Pegoraro E, Mendell JR, Bushby K, Straub V, Jain COSC (2018) Muscle MRI in patients with dysferlinopathy: pattern recognition and implications for clinical trials. J Neurol Neurosurg Psychiatr 89(10):1071–1081. https://doi.org/10.1136/jnnp-2017-317488
Evila A, Palmio J, Vihola A, Savarese M, Tasca G, Penttila S, Lehtinen S, Jonson PH, De Bleecker J, Rainer P, Auer-Grumbach M, Pouget J, Salort-Campana E, Vilchez JJ, Muelas N, Olive M, Hackman P, Udd B (2017) Targeted next-generation sequencing reveals novel TTN mutations causing recessive distal titinopathy. Mol Neurobiol 54(9):7212–7223. https://doi.org/10.1007/s12035-016-0242-3
Fischer D, Kley RA, Strach K, Meyer C, Sommer T, Eger K, Rolfs A, Meyer W, Pou A, Pradas J, Heyer CM, Grossmann A, Huebner A, Kress W, Reimann J, Schroder R, Eymard B, Fardeau M, Udd B, Goldfarb L, Vorgerd M, Olive M (2008) Distinct muscle imaging patterns in myofibrillar myopathies. Neurology 71(10):758–765. https://doi.org/10.1212/01.wnl.0000324927.28817.9b
Kesper K, Kornblum C, Reimann J, Lutterbey G, Schroder R, Wattjes MP (2009) Pattern of skeletal muscle involvement in primary dysferlinopathies: a whole-body 3.0-T magnetic resonance imaging study. Acta Neurol Scand 120(2):111–118. https://doi.org/10.1111/j.1600-0404.2008.01129.x
Lee Y, Jonson PH, Sarparanta J, Palmio J, Sarkar M, Vihola A, Evila A, Suominen T, Penttila S, Savarese M, Johari M, Minot MC, Hilton-Jones D, Maddison P, Chinnery P, Reimann J, Kornblum C, Kraya T, Zierz S, Sue C, Goebel H, Azfer A, Ralston SH, Hackman P, Bucelli RC, Taylor JP, Weihl CC, Udd B (2018) TIA1 variant drives myodegeneration in multisystem proteinopathy with SQSTM1 mutations. J Clin Invest 128(3):1164–1177. https://doi.org/10.1172/JCI97103
Mahjneh I, Bashir R, Kiuru-Enari S, Linssen W, Lamminen A, Visser M (2012) Selective pattern of muscle involvement seen in distal muscular dystrophy associated with anoctamin 5 mutations: a follow-up muscle MRI study. Neuromuscul Disord 22(2):S130–136. https://doi.org/10.1016/j.nmd.2012.02.007
Mahjneh I, Lamminen AE, Udd B, Paetau AE, Hackman P, Korhola OA, Somer HV (2004) Muscle magnetic resonance imaging shows distinct diagnostic patterns in Welander and tibial muscular dystrophy. Acta Neurol Scand 110(2):87–93. https://doi.org/10.1111/j.1600-0404.2004.00283.x
McNeill A, Birchall D, Straub V, Goldfarb L, Reilich P, Walter MC, Schramm N, Lochmuller H, Chinnery PF (2009) Lower limb radiology of distal myopathy due to the S60F myotilin mutation. Eur Neurol 62(3):161–166. https://doi.org/10.1159/000227266
Niu Z, Pontifex CS, Berini S, Hamilton LE, Naddaf E, Wieben E, Aleff RA, Martens K, Gruber A, Engel AG, Pfeffer G, Milone M (2018) Myopathy with SQSTM1 and TIA1 variants: clinical and pathological features. Front Neurol 9:147. https://doi.org/10.3389/fneur.2018.00147
Ohlsson M, Hedberg C, Bradvik B, Lindberg C, Tajsharghi H, Danielsson O, Melberg A, Udd B, Martinsson T, Oldfors A (2012) Hereditary myopathy with early respiratory failure associated with a mutation in A-band titin. Brain 135(Pt 6):1682–1694. https://doi.org/10.1093/brain/aws103
Olive M, Odgerel Z, Martinez A, Poza JJ, Bragado FG, Zabalza RJ, Jerico I, Gonzalez-Mera L, Shatunov A, Lee HS, Armstrong J, Maravi E, Arroyo MR, Pascual-Calvet J, Navarro C, Paradas C, Huerta M, Marquez F, Rivas EG, Pou A, Ferrer I, Goldfarb LG (2011) Clinical and myopathological evaluation of early- and late-onset subtypes of myofibrillar myopathy. Neuromuscul Disord 21(8):533–542. https://doi.org/10.1016/j.nmd.2011.05.002
Palmio J, Evila A, Chapon F, Tasca G, Xiang F, Bradvik B, Eymard B, Echaniz-Laguna A, Laporte J, Karppa M, Mahjneh I, Quinlivan R, Laforet P, Damian M, Berardo A, Taratuto AL, Bueri JA, Tommiska J, Raivio T, Tuerk M, Golitz P, Chevessier F, Sewry C, Norwood F, Hedberg C, Schroder R, Edstrom L, Oldfors A, Hackman P, Udd B (2014) Hereditary myopathy with early respiratory failure: occurrence in various populations. J Neurol Neurosurg Psychiatr 85(3):345–353. https://doi.org/10.1136/jnnp-2013-304965
Palmio J, Leonard-Louis S, Sacconi S, Savarese M, Penttila S, Semmler AL, Kress W, Mozaffar T, Lai T, Stojkovic T, Berardo A, Reisin R, Attarian S, Urtizberea A, Cobo AM, Maggi L, Kurbatov S, Nikitin S, Milisenda JC, Fatehi F, Raimondi M, Silveira F, Hackman P, Claeys KG, Udd B (2019) Expanding the importance of HMERF titinopathy: new mutations and clinical aspects. J Neurol 266(3):680–690. https://doi.org/10.1007/s00415-019-09187-2
Park HJ, Hong YB, Choi YC, Lee J, Kim EJ, Lee JS, Mo WM, Ki SM, Kim HI, Kim HJ, Hyun YS, Hong HD, Nam K, Jung SC, Kim SB, Kim SH, Kim DH, Oh KW, Kim SH, Yoo JH, Lee JE, Chung KW, Choi BO (2016) ADSSL1 mutation relevant to autosomal recessive adolescent onset distal myopathy. Ann Neurol 79(2):231–243. https://doi.org/10.1002/ana.24550
Park HJ, Shin HY, Kim S, Kim SH, Lee Y, Lee JH, Hong JM, Kim SM, Park KD, Choi BO, Lee JH, Choi YC (2017) Distal myopathy with ADSSL1 mutations in Korean patients. Neuromuscul Disord 27(5):465–472. https://doi.org/10.1016/j.nmd.2017.02.004
Peric S, Glumac JN, Topf A, Savic-Pavicevic D, Phillips L, Johnson K, Cassop-Thompson M, Xu L, Bertoli M, Lek M, MacArthur D, Brkusanin M, Milenkovic S, Rasic VM, Banko B, Maksimovic R, Lochmuller H, Stojanovic VR, Straub V (2017) A novel recessive TTN founder variant is a common cause of distal myopathy in the Serbian population. Eur J Hum Genet 25(5):572–581. https://doi.org/10.1038/ejhg.2017.16
Pfeffer G, Elliott HR, Griffin H, Barresi R, Miller J, Marsh J, Evila A, Vihola A, Hackman P, Straub V, Dick DJ, Horvath R, Santibanez-Koref M, Udd B, Chinnery PF (2012) Titin mutation segregates with hereditary myopathy with early respiratory failure. Brain 135(Pt 6):1695–1713. https://doi.org/10.1093/brain/aws102
Schramm N, Born C, Weckbach S, Reilich P, Walter MC, Reiser MF (2008) Involvement patterns in myotilinopathy and desminopathy detected by a novel neuromuscular whole-body MRI protocol. Eur Radiol 18(12):2922–2936. https://doi.org/10.1007/s00330-008-1071-1
Tasca G, Ricci E, Monforte M, Laschena F, Ottaviani P, Rodolico C, Barca E, Silvestri G, Iannaccone E, Mirabella M, Broccolini A (2012) Muscle imaging findings in GNE myopathy. J Neurol 259(7):1358–1365. https://doi.org/10.1007/s00415-011-6357-6
Ten Dam L, van der Kooi AJ, Rovekamp F, Linssen WH, de Visser M (2014) Comparing clinical data and muscle imaging of DYSF and ANO5 related muscular dystrophies. Neuromuscul Disord 24(12):1097–1102. https://doi.org/10.1016/j.nmd.2014.07.004
Bugiardini E, Morrow JM, Shah S, Wood CL, Lynch DS, Pitmann AM, Reilly MM, Houlden H, Matthews E, Parton M, Hanna MG, Straub V, Yousry TA (2018) The diagnostic value of MRI pattern recognition in distal myopathies. Front Neurol 9:456. https://doi.org/10.3389/fneur.2018.00456
Muller TJ, Kraya T, Stoltenburg-Didinger G, Hanisch F, Kornhuber M, Stoevesandt D, Senderek J, Weis J, Baum P, Deschauer M, Zierz S (2014) Phenotype of matrin-3-related distal myopathy in 16 German patients. Ann Neurol 76(5):669–680. https://doi.org/10.1002/ana.24255
Kraya T, Schmidt B, Muller T, Hanisch F (2015) Impairment of respiratory function in late-onset distal myopathy due to MATR3 mutation. Muscle Nerve 51(6):916–918. https://doi.org/10.1002/mus.24603
Barp A, Malfatti E, Metay C, Jobic V, Carlier RY, Laforet P (2018) The first French case of MATR3-related distal myopathy: Clinical, radiological and histopathological characterization. Rev Neurol (Paris). https://doi.org/10.1016/j.neurol.2017.08.004
Feit H, Silbergleit A, Schneider LB, Gutierrez JA, Fitoussi RP, Reyes C, Rouleau GA, Brais B, Jackson CE, Beckmann JS, Seboun E (1998) Vocal cord and pharyngeal weakness with autosomal dominant distal myopathy: clinical description and gene localization to 5q31. Am J Hum Genet 63(6):1732–1742. https://doi.org/10.1086/302166
Palmio J, Evila A, Bashir A, Norwood F, Viitaniemi K, Vihola A, Huovinen S, Straub V, Hackman P, Hirano M, Bushby K, Udd B (2016) Re-evaluation of the phenotype caused by the common MATR3 p.Ser85Cys mutation in a new family. J Neurol Neurosurg Psychiatr 87(4):448–450. https://doi.org/10.1136/jnnp-2014-309349
Senderek J, Garvey SM, Krieger M, Guergueltcheva V, Urtizberea A, Roos A, Elbracht M, Stendel C, Tournev I, Mihailova V, Feit H, Tramonte J, Hedera P, Crooks K, Bergmann C, Rudnik-Schoneborn S, Zerres K, Lochmuller H, Seboun E, Weis J, Beckmann JS, Hauser MA, Jackson CE (2009) Autosomal-dominant distal myopathy associated with a recurrent missense mutation in the gene encoding the nuclear matrix protein, matrin 3. Am J Hum Genet 84(4):511–518. https://doi.org/10.1016/j.ajhg.2009.03.006
Yamashita S, Mori A, Nishida Y, Kurisaki R, Tawara N, Nishikami T, Misumi Y, Ueyama H, Imamura S, Higuchi Y, Hashiguchi A, Higuchi I, Morishita S, Yoshimura J, Uchino M, Takashima H, Tsuji S, Ando Y (2015) Clinicopathological features of the first Asian family having vocal cord and pharyngeal weakness with distal myopathy due to a MATR3 mutation. Neuropathol Appl Neurobiol 41(3):391–398. https://doi.org/10.1111/nan.12179
Khaleeli AA, Edwards RH, Gohil K, McPhail G, Rennie MJ, Round J, Ross EJ (1983) Corticosteroid myopathy: a clinical and pathological study. Clin Endocrinol (Oxf) 18(2):155–166. https://doi.org/10.1111/j.1365-2265.1983.tb03198.x
May DA, Disler DG, Jones EA, Balkissoon AA, Manaster BJ (2000) Abnormal signal intensity in skeletal muscle at MR imaging: patterns, pearls, and pitfalls. Radiographics 20:S295–315. https://doi.org/10.1148/radiographics.20.suppl_1.g00oc18s295
Burian E, Syvari J, Holzapfel C, Drabsch T, Kirschke JS, Rummeny EJ, Zimmer C, Hauner H, Karampinos DC, Baum T, Franz D (2018) Gender- and age-related changes in trunk muscle composition using chemical shift encoding-based water(-)fat MRI. Nutrients 10(12):1972. https://doi.org/10.3390/nu10121972
Yin L, Xie ZY, Xu HY, Zheng SS, Wang ZX, Xiao JX, Yuan Y (2019) T2 Mapping and fat quantification of thigh muscles in children with Duchenne muscular dystrophy. Curr Med Sci 39(1):138–145. https://doi.org/10.1007/s11596-019-2012-8
Chardon JW, Diaz-Manera J, Tasca G, Bonnemann CG, Gomez-Andres D, Heerschap A, Mercuri E, Muntoni F, Pichiecchio A, Ricci E, Walter MC, Hanna M, Jungbluth H, Morrow JM, Fernandez-Torron R, Udd B, Vissing J, Yousry T, Quijano-Roy S, Straub V, Carlier RY, Group M-MW (2019) MYO-MRI diagnostic protocols in genetic myopathies. Neuromuscul Disord 29(11):827–841. https://doi.org/10.1016/j.nmd.2019.08.011
Penisson-Besnier I, Dumez C, Chateau D, Dubas F, Fardeau M (1998) Autosomal dominant late adult onset distal leg myopathy. Neuromuscul Disord 8(7):459–466. https://doi.org/10.1016/s0960-8966(98)00063-7
Penisson-Besnier I, Talvinen K, Dumez C, Vihola A, Dubas F, Fardeau M, Hackman P, Carpen O, Udd B (2006) Myotilinopathy in a family with late onset myopathy. Neuromuscul Disord 16(7):427–431. https://doi.org/10.1016/j.nmd.2006.04.009
Selcen D, Engel AG (2004) Mutations in myotilin cause myofibrillar myopathy. Neurology 62(8):1363–1371. https://doi.org/10.1212/01.wnl.0000123576.74801.75
Lindberg C, Borg K, Edstrom L, Hedstrom A, Oldfors A (1991) Inclusion body myositis and Welander distal myopathy: a clinical, neurophysiological and morphological comparison. J Neurol Sci 103(1):76–81. https://doi.org/10.1016/0022-510x(91)90287-h
von Tell D, Somer H, Udd B, Edstrom L, Borg K, Ahlberg G (2002) Welander distal myopathy outside the Swedish population: phenotype and genotype. Neuromuscul Disord 12(6):544–547. https://doi.org/10.1016/s0960-8966(01)00338-8
Udd B, Partanen J, Halonen P, Falck B, Hakamies L, Heikkila H, Ingo S, Kalimo H, Kaariainen H, Laulumaa V et al (1993) Tibial muscular dystrophy. Late adult-onset distal myopathy in 66 Finnish patients. Arch Neurol 50(6):604–608. https://doi.org/10.1001/archneur.1993.00540060044015
Acknowledgements
Open Access funding provided by Projekt DEAL. The authors would like to thank all patients and families for their willingness to participate in this study.
Funding
This work was financially supported by the Martin Luther University of Halle-Wittenberg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
AM, TK, FK, TM and DS report no conflicts of interest. SZ received personal fees from Sanofi Genzyme, outside the submitted work.
Ethical approval
This study was approved by the institutional research committee at Martin-Luther-University of Halle-Wittenberg.
Informed consent
Informed consent was obtained from all patients included in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mensch, A., Kraya, T., Koester, F. et al. Whole-body muscle MRI of patients with MATR3-associated distal myopathy reveals a distinct pattern of muscular involvement and highlights the value of whole-body examination. J Neurol 267, 2408–2420 (2020). https://doi.org/10.1007/s00415-020-09862-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09862-9