Abstract
Background
Patients with pulmonary arterial hypertension (PAH) and distal chronic thromboembolic pulmonary hypertension (CTEPH) who still reveal risk factors of worse prognosis on double combination therapy may benefit from add-on therapy with the novel oral selective prostacyclin receptor agonist selexipag.
Methods
We reviewed all patients with PAH/distal CTEPH in the Zurich cohort who received selexipag as add-on to oral combination therapy and retrieved New York Heart Association (NYHA) functional class, 6-min walk distance (6MWD), NT-pro-BNP, quality of life questionnaires (CAMPHOR and EuroQoL), tricuspid pressure gradient (TPG) by echocardiography and cardiopulmonary exercise test parameters (power output and oxygen uptake).
Results
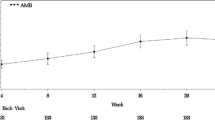
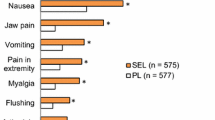
Twenty-three patients with PAH/CTEPH (20/3), 14 females, median (quartiles) age 56 (46; 66) years received an oral triple therapy containing selexipag at a median dose of 2000 (1600; 3100) mcg during 221 (113; 359) days. The following parameters were stabilized from baseline to last FU: 6MWD (440 (420; 490) to 464 (420; 526) m), NYHA class (three to two), NT-pro-BNP (326 (167; 1725) to 568 (135; 1856) ng/l), TPG, power output, and oxygen uptake. Quality of life reflected by the CAMPHOR and EuroQoL improved.
Conclusions
Early initiation of triple oral combination therapy including selexipag in PAH/CTEPH with intermediate risk factor profile may help to stabilize functional class, exercise performance, and pulmonary hemodynamics in a real-life setting and potentially improves quality of life. Whether these beneficial effects can be truly attributed to the addition of selexipag should be addressed in future randomized controlled trials.


Similar content being viewed by others
References
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A et al (2015) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 37(1):67–119
Wilkens H, Konstantinides S, Lang I, Bunck AC, Gerges M, Gerhardt F et al (2016) Chronic thromboembolic pulmonary hypertension: recommendations of the Cologne Consensus Conference 2016. Dtsch Med Wochenschr 141(S01):S62–S69
Ulrich S, Fischler M, Speich R, Popov V, Maggiorini M (2006) Chronic thromboembolic and pulmonary arterial hypertension share acute vasoreactivity properties. Chest 130(3):841–846
Cenedese E, Speich R, Dorschner L, Ulrich S, Maggiorini M, Jenni R et al (2006) Measurement of quality of life in pulmonary hypertension and its significance. Eur Respir J 28(4):808–815
Cannon JE, Pepke-Zaba J (2013) Is distal chronic thromboembolic pulmonary hypertension treatable with PAH targeted drugs? Semin Respir Crit Care Med 34(5):620–626
Voelkel NF, Cool C (2004) Pathology of pulmonary hypertension. Cardiol Clin 22(3):343–351
Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV et al (2015) Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 373(9):834–844
Galie N, Muller K, Scalise AV, Grunig E (2015) PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur Respir J 45(5):1314–1322
Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA et al (2013) Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 369(9):809–818
Bartenstein P, Saxer S, Appenzeller P, Lichtblau M, Schwarz EI, Ulrich S (2018) Risk factor profiles achieved with medical therapy in prevalent patients with pulmonary arterial and distal chronic thromboembolic pulmonary hypertension. Respiration 96(1):127–137
Sitbon O, Sattler C, Bertoletti L, Savale L, Cottin V, Jais X et al (2016) Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J 47(6):1727–1736
Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC et al (2013) Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 369(4):330–340
Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlocai K, Galie N et al (2012) Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J 40(4):874–880
Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galie N et al (2015) Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 373(26):2522–2533
Gaine S, Chin K, Coghlan G, Channick R, Di Scala L, Galie N et al (2017) Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J 50(2):1602493
Mueller-Mottet S, Stricker H, Domenighetti G, Azzola A, Geiser T, Schwerzmann M et al (2015) Long-term data from the Swiss pulmonary hypertension registry. Respiration. 89(2):127–140
Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D et al (2011) Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 124(18):1973–1981
Evangelista A, Flachskampf F, Lancellotti P, Badano L, Aguilar R, Monaghan M et al (2008) European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur J Echocardiogr 9(4):438–448
Kylhammar D, Kjellstrom B, Hjalmarsson C, Jansson K, Nisell M, Soderberg S et al (2017) A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 39(47):4175–4181
Boucly A, Weatherald J, Savale L, Jais X, Cottin V, Prevot G et al (2017) Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 50(2):1700889
Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N et al (2017) Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 50(2):1700740
Coghlan JG, Channick R, Chin K, Di Scala L, Galie N, Ghofrani HA et al (2018) Targeting the prostacyclin pathway with selexipag in patients with pulmonary arterial hypertension receiving double combination therapy: insights from the randomized controlled GRIPHON study. Am J Cardiovasc Drugs 18(1):37–47
McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S et al (2013) Treatment goals of pulmonary hypertension. J Am Coll Cardiol 62(25 Suppl):D73–D81
Deboeck G, Scoditti C, Huez S, Vachiery JL, Lamotte M, Sharples L et al (2012) Exercise testing to predict outcome in idiopathic versus associated pulmonary arterial hypertension. Eur Respir J 40(6):1410–1419
McKenna SP, Doughty N, Meads DM, Doward LC, Pepke-Zaba J (2006) The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR): a measure of health-related quality of life and quality of life for patients with pulmonary hypertension. Qual Life Res 15(1):103–115
Cima K, Twiss J, Speich R, McKenna SP, Grunig E, Kahler CM et al (2012) The German adaptation of the Cambridge pulmonary hypertension outcome review (CAMPHOR). Health Qual Life Outcomes 10(1):110
Meads DM, McKenna SP, Doughty N, Das C, Gin-Sing W, Langley J et al (2008) The responsiveness and validity of the CAMPHOR utility index. Eur Respir J 32(6):1513–1519
Brooks R (1996) EuroQol: the current state of play. Health Policy 37(1):53–72
Funding
The study was funded by grants for the Zurich PH cohort from the Zurich Lung.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declares any conflict of interest in relation to the present work. SU received grant money from the Swiss National Science Foundation, Zurich Lung, Actelion SA, Bayer SA, Orpha Swiss. SU; EIS, SS, ML and CB received travel support and lecture fees from Actelion SA, Bayer SA, MSD SA and Orpha Swiss.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This retrospective cohort study complied with the ethical laws in Switzerland and all patients have signed informed consent for the Zurich PH cohort study (KEK 2014-0214).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berlier, C., Schwarz, E.I., Saxer, S. et al. Real-Life Experience with Selexipag as an Add-On Therapy to Oral Combination Therapy in Patients with Pulmonary Arterial or Distal Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Analysis. Lung 197, 353–360 (2019). https://doi.org/10.1007/s00408-019-00222-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-019-00222-7