Abstract
Introduction
The main goal of this retrospective cohort study is the assessment of the effects of administration of recombinant-hCG (r-hCG) versus urinary-hCG (u-hCG) on follicular fluid (FF) composition of women who underwent in vitro fertilization (IVF) treatments.
Materials and methods
We selected 70 patients with infertility attributable to tubal diseases, unexplained infertility, and male factor. Metabolomics analysis of their FFs was performed by 1H nuclear magnetic resonance (1H NMR) spectroscopy in combination with multivariate analysis to interpret the spectral data. Univariate statistical analysis was applied to investigate the possible correlations between clinical parameters and between clinical parameters and metabolites identified by NMR.
Results
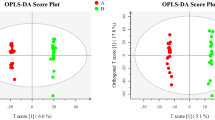
According to the type of hCG used, significant differences were detected in FFs of women with male factor and unexplained infertility, both in qualitative and quantitative terms, for some metabolites as cholesterol, citrate, creatine, β-hydroxybutyrate, glycerol, lipids, amino acids (Glu, Gln, His, Val, Lys) and glucose. No significant difference was observed in women with tubal diseases. Besides, the number of MII oocytes in the u-hCG-treated groups correlates positively with glutamate in tubal disease and with glycerol in unexplained infertility. In the r-hCG-treated groups, the number of MII oocytes correlates positively with lipid in tubal disease, positively with citrate and negatively with glucose in male infertility.
Conclusions
Metabolite composition of FF changes according to different type of hCG treatment and this can be related to oocyte development and subsequent outcome. According to the data of this study, different types of hCG should be used in relation to the diagnosis of infertility to obtain better results in inducing oocyte maturation in women undergoing IVF.




Similar content being viewed by others
Abbreviations
- ART:
-
Assisted reproductive technologies
- β-HB:
-
β-Hydroxybutyrate
- BMI:
-
Body mass index
- CPMG:
-
Carr–Purcell–Meiboom–Gill
- D2O:
-
Deuterated water
- FF:
-
Follicular fluid
- FSH:
-
Follicle-stimulating hormone
- GnRH:
-
Gonadotropin-releasing hormone
- hCG:
-
Human chorionic gonadotropin
- ICSI:
-
Intracytoplasmic sperm injection
- IVF:
-
In vitro fertilization
- MAP:
-
Medically assisted procreation
- NMR:
-
Nuclear magnetic resonance
- PCA:
-
Principal component analysis
- PLS-DA:
-
Partial least squares discriminant analysis
- r-hCG:
-
Recombinant human chorionic gonadotropin
- TSP:
-
3-Trimethylsilyl propionic acid-d4 sodium salt
- u-hCG:
-
Urinary human chorionic gonadotropin
- VIP:
-
Variable importance in the projection
References
Jungheim ES, Meyer M, Broughton DE (2015) Best practices for controlled ovarian stimulation. Semin Reprod Med 33(2):77–82
Abbara A, Clarke SA, Dhillo WS (2017) Novel concepts for inducing final oocyte maturation in In vitro fertilization treatment. Endocr Rev 39(5):593–628
Haahr T, Esteves SC, Humadain P (2018) Individualized controlled ovarian stimulation in expected poor-responders: an update. Reprod Biol Endocrinol 16(1):20. https://doi.org/10.1186/s12958-018-0342-1
Cole LA (2010) Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol 8:102. https://doi.org/10.1186/1477-7827-8-102
Nwabuobi C, Arlier S, Schatz F, Guzeloglu-Kayisli O, Lockwood CJ, Kayisli UA (2017) hCG: Biological functions and clinical applications. Int J Mol Sci. https://doi.org/10.3390/ijms18102037
Fournier T, Guibourdenche J, Evain-Brion D (2015) Review: hCGs: different sources of production, different glycoforms and functions. Placenta 36. Suppl 1:S60–S65
Fournier T (2016) Human chorionic gonadotropin: different glycoforms and biological activity depending on its source of production. Ann d’Endocrinologie 77:75–81
Gunnala V, Melnick A, Irani M, Reichman D, Schattman G, Davis O, Rosenwaks Z (2017) Sliding scale HCG trigger yields equivalent pregnancy outcomes and reduces ovarian hyperstimulation syndrome: analysis of 10,427 IVF-ICSI cycles. PLoS ONE 12(4):e0176019. https://doi.org/10.1371/journal.pone.0176019
Leāo RB, Esteves SC (2014) Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech. Clinics 69(4):279–293
Gervais A, Hammel YA, Pelloux S, Lepage P, Baer G, Carte N, Sorokine O, Strub JM, Koerner R, Leize E, Van Dorsselaer A (2003) Glycosylation of human recombinant gonadotropins: characterization and batch-to-batch consistency. Glycobiology 13(3):179–189. https://doi.org/10.1093/glycob/cwg020
Stenman UH, Tiitinen A, Alfthan H, Valmu L (2006) The classification, functions and clinical use of different isoforms of HCG. Human Reprod Update 12(6):769–784. https://doi.org/10.1093/humupd/dml029
The European Recombinant Human Chorionic Gonadotropin Study Group (2000) Induction of final follicular maturation and early luteinization in women undergoing ovulation induction for assisted reproduction treatment-recombinant HCG versus urinary HCG. Hum Reprod 15(7):1446–1451
Al-Inany H, Aboulghar MA, Mansour RT, Proctor M (2005) Recombinant versus urinary gonadotropins for triggering ovulation in assisted conception. Hum Reprod 20(8):2061–2073
Uhler ML, Beltsos AN, Grotjan HE, Lederer KJ, Lifchez AS (2006) Age-matched comparison of recombinant and urinary HCG for final follicular maturation. Reprod BioMed Online 13(3):315–332
Eftekhar M, Khalili MA, Rahmani E (2012) The efficacy of recombinant versus urinary HCG in ART outcome. Iran J Reprod Med 10(6):543–548
Bellavia M, de Geyter C, Streuli I, Ibecheole V, Birkhäuser MH, Cometti BPS, de Ziegler D (2013) Randomized controlled trial comparing highly purified (HP-hCG) and recombinant hCG (r-hCG) for triggering ovulation in ART. Gynecol Endocrinol 29(2):93–97
Madani T, Mohammadi Yeganeh L, Ezabadi Z, Hasani F, Chehrazi M (2013) Comparing the efficacy of urinary and recombinant hCG on oocyte/follicle ratio to trigger ovulation in women undergoing intracytoplasmic sperm injection cycles: a randomized controlled trial. J Assist Reprod Genet 30(2):239–245
Youssef MA, Al-Inany HG, Aboulghar M, Mansour R, Abou-Setta AM (2011) Recombinant versus urinary human chorionic gonadotropin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003719.pub3
Youssef MA, Abou-Setta AM, Lam WS (2016) Recombinant versus urinary human chorionic gonadotropin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane database Syst Rev 4:3719. https://doi.org/10.1002/14651858.CD003719.pub4
Bagchus W, Wolna P, Uhl W (2017) Single-dose pharmacokinetic study comparing the pharmacokinetics of recombinant human chorionic gonadotropin in healthy Japanese and Caucasian women and recombinant human chorionic gonadotropin and urinary human chorionic gonadotropin in healthy Japanese women. Reprod Med Biol 17(1):52–58
Da Broi MG, Giorgi VSI, Wang F, Keefe DL, Albertini D, Navarro PA (2018) Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod Genet 35(5):735–751. https://doi.org/10.1007/s10815-018-1143-3
La Marca A, Grisendi V, Giulini S, Argento C, Tirelli A, Dondi G et al (2013) Individualization of the FSH starting dose in IVF/ICSI cycles using the antral follicle count. J Ovarian Res 6:11. https://doi.org/10.1186/1757-2215-6-11
Piñero-Sagredo E, Nunes S, de los Santos MJ, Celda B, Esteve V (2010) NMR metabolic profile of human follicular fluid. NMR Biomed 23:485–495
Baskind NE, McRae C, Sharma V, Fisher J (2011) Understanding subfertility at a molecular level in the female through the application of nuclear magnetic resonance (NMR) spectroscopy. Hum Reprod Update 17(2):228–241
Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L (2012) An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil Steril 97(5):1078–1084
O’Gorman A, Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M et al (2013) Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 146(4):389–395
Karaer A, Tuncay G, Mumcu A, Dogan B (2018) Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Syst Biol Reprod Med 28:1–9
Castiglione Morelli MA, Iuliano A, Schettini SCA, Petruzzi D, Ferri A, Colucci P, Viggiani L, Cuviello F, Ostuni A (2018) NMR metabolomics study of follicular fluid in women with cancer resorting to fertility preservation. J Assist Reprod Genet 35(11):2063–2070
Castiglione Morelli MA, Iuliano A, Schettini SCA, Petruzzi D, Ferri A, Colucci P, Viggiani L, Cuviello F, Ostuni A (2019) NMR metabolic profiling of follicular fluid for investigating the different causes of female infertility: a pilot study. Metabolomics 15(2):19
Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P (2009) Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol 7:40. https://doi.org/10.1186/1477-7827-7-40
Bracewell-Milnes T, Saso S, Abdalla H, Nikolau D, Norman-Taylor J, Johnson M et al (2017) Metabolomics as a tool to identify biomarkers to predict and improve outcomes in reproductive medicine: a systematic review. Hum Reprod Update 23(6):723–736
Santonastaso M, Pucciarelli A, Costantini S, Caprio F, Sorice A, Capone F et al (2017) Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol Biosyst 13:1213–1222
Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB (2015) Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril 103(2):303–316
Hemmings KE, Maruthini D, Vyjayanthi S, Hogg JE, Balen AH, Campbell BK, Leese HJ, Picton HM (2013) Amino acid turnover by human oocytes is influenced by gamete developmental competence, patient characteristics and gonadotropin treatment. Hum Reprod 28(4):1031–1044
Matoba S, Bender K, Fahey AG, Mamo S, Brennan L, Lonergan P, Fair T (2014) Predictive value of bovine follicular components as markers of oocyte developmental potential. Reprod Fertil Dev 26:337–345
Sutton-McDowall ML, Gilchrist RB, Thompson JG (2010) The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139:685–695
Santi D, Casarini L, Alviggi C, Simoni M (2017) Efficacy of follicle-stimulating hormone (FSH) alone, FSH + luteinizing hormone, human menopausal gonadotropin or FSH + human chorionic gonadotropin on assisted reproductive technology outcomes in the "Personalized" medicine era: a meta-analysis. Front Endocrinol 8:114. https://doi.org/10.3389/fendo.2017.00114
Mol BW, Bossuyt PM, Sunkara SK, Garcia Velasco JA, Venetis C, Sakkas D, Lundin K, Simon C, Taylor HS, Wan R, Longobardi S, Cottell E, D'Hooghe T (2018) Personalized ovarian stimulation for assisted reproductive technology: study design considerations to move from hype to added value for patients. Fertil Steril 109(6):968–979
Funding
The authors received no financial support for this study.
Author information
Authors and Affiliations
Contributions
SS, AI, and AO designed the study. AI selected the patients and executed oocyte retrieval. DP executed oocyte retrieval. AF identified oocytes in follicular fluid and executed their fertilization. PC selected the follicular fluids to be used for metabolomic analyses. CM performed the analysis of NMR data and multivariate analysis. LV ran the NMR experiments. AO performed statistical analysis. AO, AI, and CM were responsible for conducting the study and writing the manuscript which was critically discussed, edited and approved by all co-authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local ethical committee, Comitato Etico Unico Regionale per la Basilicata (CEUR, approval number: 2015/00585), and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by CEUR on November 27, 2015.
Consent for publication
Written informed consent for publication was obtained from the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors consider that the first two authors should be regarded as joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castiglione Morelli, M.A., Iuliano, A., Schettini, S.C.A. et al. Metabolic changes in follicular fluids of patients treated with recombinant versus urinary human chorionic gonadotropin for triggering ovulation in assisted reproductive technologies: a metabolomics pilot study. Arch Gynecol Obstet 302, 741–751 (2020). https://doi.org/10.1007/s00404-020-05609-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05609-z