Abstract
Purpose
To compare the influence of Cimicifuga racemosa extract (CR, Ze 450) and menopausal hormone therapy (MHT) on metabolic parameters and body weight in symptomatic menopausal women.
Methods
In this monocentric retrospective cohort study, women over 40 years old with a first consultation between 2009 and 2016 were screened. Included in the final analysis were women treated with either MHT or CR and having at least one follow-up consultation. Metabolic serum parameters (lipids, glucose, insulin, and HOMA-IR), body weight, and menopausal symptoms [Menopause Rating Scale (MRS)-II] were the main outcome measures. Statistical analysis by uni- and multi-variable linear mixed-effects regression models assuming a linear effect of time.
Results
174 women were included in the final analysis (CR n = 32, MHT n = 142). There was no difference between the groups regarding baseline characteristics (age, BMI, serum metabolic parameters, hormones, and blood pressure) and total MRS-II score, while reproductive stage differed significantly with more postmenopausal women treated with CR (83%) than MHT (55%) (p = 0.038). Median follow-up time was 12 months. In both groups, metabolic serum parameters and body weight did not change over the follow-up period, while total and MRS-II subscores improved.
Conclusion
Menopausal symptoms improved significantly in both groups (MHT and CR), while serum metabolic parameters and body weight did not change in MHT- or CR-treated women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The menopausal transition and ageing are accompanied by body weight increase of about 0.5 kg annually [1,2,3]. Weight gain, especially during menopause, has been significantly associated with increased risk of several diseases [2]. Furthermore, body composition changes with visceral fat depots increasing and lean body mass decreasing [4] often accompanied by adverse changes in metabolic parameters [5]. Menopausal hormone therapy (MHT) is usually indicated for menopausal symptom relief, but also has a positive impact on body weight, body composition, and metabolic parameters [6,7,8].
Black cohosh (Cimicifuga racemosa, CR) is an alternative treatment for menopausal hot flushes [9, 10]. Its precise mechanism of action is controversial. For hot flush reduction, CR is thought to modulate the neurotransmitter signaling pathways in the brain and not to exert estrogenic effects [11,12,13]. In vitro, the Black cohosh dry extract Ze 450 [(DER 4.5–8.5:1), extraction solvent ethanol 60% (V/V)] has been shown to dose-dependently activate AMP-activated protein kinase (AMPK), an activity regulator of different key enzymes involved in lipid synthesis (HMG-CoA reductase and acetyl-CoA carboxylase), and some glucose transporters (Glut 1/4) [14]. Herein, AMPK activation was more pronounced with Ze 450 than metformin treatment. These findings were confirmed in ob/ob mice showing a significant reduction of water intake and a normalization of the glucose and insulin response to a glucose challenge test [14]. Thus, Ze 450 may improve metabolic control via improving insulin receptor sensitivity. The aim of this monocentric retrospective study was to compare the impact of the Black cohosh dry extract Ze 450 versus MHT on body weight and metabolic parameters, such as serum lipids, glucose, insulin, and HOMA-IR in symptomatic menopausal women.
Methods
Study design
CIMBOLIC is an investigator-initiated monocentric retrospective observational study. Coded health-related data of all patients above age 40 presenting for first-time consultation at the Menopause Centre of the Department of Obstetrics and Gynecology, Inselspital Bern, between 01.04.2009 and 31.12.2016 were abstracted from medical records. Routine assessment comprised personal and family history including reproductive age [15], medication, lifestyle factors (smoking, alcohol), questionnaire Menopause Rating Scale (MRS)-II [16], anthropological parameters (body weight, height, body mass index, blood pressure, and waist circumference), laboratory blood tests, and risk evaluation of certain non-communicable diseases (cardiovascular disease, breast cancer, osteoporosis), respectively (supplementary file 1). The selection of the treatment option (CR or MHT) was based on the above-described routine assessment by the physician, a benefit–risk evaluation, and the patients wish at the time of consultation. For the final analysis, all patients treated with either Ze 450 (Cimifemin® forte = 13 mg dry extract, Cimifemin® uno = 6.5 mg dry extract or Climavita® forte = 13 mg dry extract) or any MHT (but not both) during the follow-up period and having at least one follow-up visit including blood tests were eligible. The study was approved by the cantonal ethics committee (Kantonale Ethik-kommission Bern (KEK) Switzerland No 2016-01179).
Data sources
Data from medical records were transferred to a REDCap (Research Electronic Data Capture) database. Generation, transmission, storage, and analysis of health-related personal data within this project were according to the current Swiss legal requirements for data protection and were performed according to the Ordinance HRO Art. 5. This observational study is reported according to the STROBE statement (see www.strobe-statement.org).
Statistical methods
Patients were grouped according to whether they were treated with Ze 450 or MHT during the follow-up period. Continuous and categorical variables are presented by group using median with lower and upper quartiles (lq, uq), and number and percentage of patients, respectively. Baseline characteristics between groups were compared using Wilcoxon rank-sum tests and Fisher’s exact tests for continuous and categorical variables, respectively. Linear mixed-effects regression models were used for the analysis of a change over time within and between groups. The endpoint values at baseline and each follow-up were used as dependent variable and the treatment group (i.e., MHT or Ze 450), the time, and the interaction of time and group as fixed explanatory variables. A random intercept was included for each patient to account for intra-patient correlations. The models were fitted with restricted maximum likelihood, the degrees of freedom were calculated using the Satterthwaite approximation and 95% confidence intervals (95% CI) based on the t distribution. Marginal effects for each group were calculated using Stata’s margin command and expressed as mean change per year with 95% CI and a p value. The difference between groups (i.e., the interaction term of group and time) was expressed as mean difference per year (Ze 450 minus MHT) with 95% CI and a p value. For the main analysis, all patients in the full analysis set were included, regardless whether a baseline and a follow-up assessment were available for a specific endpoint. For a sensitivity analysis, only patients with a baseline and at least one follow-up measurement were included (referred to as complete case analysis). We used a multi-variable linear mixed-effects regression to adjust for confounding. All baseline variables with a p value < 0.2 in the baseline comparison and less than 30% missingness in both groups were included as covariates. An overall control of the type I error rate was not done because of the explorative nature of the study. The reported p values were not corrected for multiplicity and have to be interpreted accordingly. All analyses were done with Stata [StataCorp. Stata Statistical Software: Release 14. StataCorp, College Station, TX: StataCorp LP., 2015.] or R [R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2016. URL http://www.R-project.org. ISBN 3-900051-07-0.].
Results
Participants
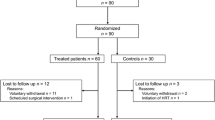
Figure 1 displays the patient flow. Of 769 patients screened, 174 were eligible for the final analysis. Of those, 32 were treated with Ze 450 and 142 with MHT, respectively. Current systemic MHT regimens comprised mainly estrogen–progestogen combinations (n = 89; 63%), while the remainder received either estrogens only, progestogens only, estrogen–androgen combinations, or tibolone, respectively. Only few patients in the Ze 450 group (n = 3; 9%) but most in the MHT group (n = 103; 73%) had a history of MHT use. However, at first consultation few patients were using Ze 450 (n = 8; 25%) or MHT (n = 49; 35%), respectively. All Black cohosh user had a Ze 450 product. Of those, the majority had a daily dosage of Ze 450 at 13 mg dry extract (n = 25; 78%; recommended dose 1 tablet daily).
Subjects’ characteristics at baseline
Table 1 presents subjects’ anthropological, reproductive, and lifestyle parameters at baseline. There was no significant intergroup difference but for reproductive stage (p = 0.038). The majority of women treated with Ze 450 were postmenopausal (n = 24; 83%), while only one in two women treated with MHT fell into this category (n = 66; 55%). About one-third of MHT-treated women were either premenopausal (n = 21; 17%) or in the early menopausal transition (n = 23; 19%). In both groups, mean follow-up was 12 months (Ze 450 lq, uq 12.0, 24.0; MHT lq, uq 12.0, 36.0; p = 0.91). There was a significant difference in the number of follow-up visits (p = 0.011). 88% (Ze 450) and 61% (MHT) of subjects had only one follow-up visit. A few women treated with Ze 450 had two (n = 2; 6%), three (n = 1; 3%), or four (n = 1; 3%) follow-up visits, compared to 41 (29%) with two, 12 (8%) with three, and 3 (2%) with four follow-up visits in the MHT group.
Table 2 presents the type and intensity of menopausal symptoms at baseline, assessed by the questionnaire MRS-II. In both groups, median intensity of overall menopausal symptoms (MRS-II total score) at baseline was moderate (defined as score 9–16) to severe (defined as score ≥ 17) (p = 0.46). When differentiating between menopausal symptom subdomains, both groups suffered from moderate vegetative symptoms (defined as score 5–8) (p = 0.10) and moderate (defined as score 2–3)-to-severe (defined as score ≥ 4) urogenital symptoms (p = 0.89), respectively. Psychological menopausal symptoms were moderate (defined as score 4–6) at baseline in both groups with women choosing MHT later on being slightly but significantly more affected than women choosing Ze 450 (p = 0.011).
Change of menopausal symptoms, body weight, and metabolic parameters over time
We did not find any evidence for a within-group change in body weight, carbohydrate metabolism (glucose, insulin, and HOMA-IR), and serum lipids (total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides), neither in women treated with Ze 450 nor with MHT (Table 3). For MHT, we found a significant improvement over time of the MRS-II total score (− 0.99 [95% CI − 1.42, − 0.55] per year; p < 0.0001), MRS-II vegetative subscore (− 0.24 [95% CI − 0.45, − 0.03] per year; p = 0.023), MRS-II psychological subscore (− 0.48 [95% CI − 0.71, − 0.25]; p < 0.0001), and MRS-II urogenital subscore (− 0.28 [95% CI − 0.45, − 0.11]; p = 0.001). Similarly, treatment with Ze 450 significantly improved the MRS-II vegetative subscore (− 0.81 [95% CI − 1.57, − 0.04]; p = 0.039) and urogenital subscore (− 0.64 [95% CI − 1.26, − 0.01]; p = 0.045) (Table 3). MRS-II total score had the same trend (− 1.43 [95% CI − 3.16, 0.30]; p = 0.11). Between-group comparisons did not reveal significant differences for any endpoint (Table 3).
To adjust for potential confounding, we included baseline variables with a p value < 0.2 in the baseline comparisons and less than 30% missingness (diastolic blood pressure, age at menarche, reproductive stage, smoking status, MRS-II vegetative subscore, and MRS-II psychological subscore) in multi-variable models (Table 4). Results were similar as in the univariable models. For MHT, we found evidence for a change of the MRS-II total score (− 0.87 [95% CI − 1.26, − 0.48] per year; p < 0.0001), the psychological subscore (− 0.42 [95% CI − 0.60, − 0.24] per year; p < 0.0001), and the urogenital subscore (− 0.24 [95% CI − 0.42 to − 0.07] per year; p = 0.005). For Ze 450, we found some evidence for a change of the total score (− 1.82 [95% CI − 3.65, 0.02]; p = 0.05) and the urogenital subscore (− 0.79 [95% CI − 1.60, 0.02]; p = 0.05). We did not find any evidence for a between-group difference. Furthermore, we did not find within or between-group changes for body weight and serum metabolic parameter (total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, fasting glucose, insulin, and HOMA-IR) (Table 4).
Discussion
This monocentric, retrospective, observational study found that (1) both the Black cohosh dry extract Ze 450 and MHT markedly reduced menopausal symptoms over time without displaying significant intergroup differences with (2) MHT having a significant beneficial impact on psychological and urogenital symptom relief. Furthermore (3) in contrast to our expectations of weight gain during menopause [1, 2], we did not find any evidence for a change in body weight and metabolic parameters (serum lipids, carbohydrate metabolism) over time. However, uncertainty of the estimated effects was large. Finally, (4) potential confounders did not alter the results.
Clearly, this study has some limitations. The study was explorative and we tested many hypotheses without controlling the overall type I error rate—all results have to be interpreted accordingly. As this was a retrospective observational study, treatment indication, dosage, and duration were heterogenous. Sample size per group was small, the number of missing data was quite high, and we did not have an untreated control group. The power to detect differences between groups was small and not finding any does not provide evidence for the equality of the treatments. The pre-specified power analysis showed that 150 and 850 patients treated with Ze 450 and MHT, respectively, would be necessary to detect realistic effect sizes of 0.25 with a power of 80%. The absence of an untreated control group makes it impossible to exclude a placebo effect, in particular since we only found effects on subjective endpoints (the MRS scores). We assumed linear effects of time on all outcomes. This assumption is unlikely to be true but seems reasonable over the rather short follow-up time. The small sample size did not allow us to study the effect of time in more detail. On the other hand, the strength of the study was its real-life character when treatment initiation depends on multiple factors and does not exclude anyone like in prospective randomized-controlled trials. These results can, therefore, be transferred into the daily life situation of gynecologists working in outpatient clinics and private practices.
Interestingly, serum vitamin D3 levels were significantly lower in Ze 450 than in MHT-treated women. According to national guidelines, mean serum vitamin D3 levels were below the recommended serum levels within the Black cohosh cohort but within the recommended range in MHT-treated women (> 50 mmol/l) [17]. However, vitamin D supplementation has not been shown to have an impact on, e.g., glycemia or insulin resistance [18]. The assessment of vitamin D was incomplete (63% and 75% of the women had an assessment at baseline in Ze 450 and MHT group, respectively) and we did not include it in the multi-variable model.
The observation of a beneficial impact of the Black cohosh dry extract Ze 450 on menopausal symptoms is shown in an RCT [19] and supports a previous systematic review showing a reduction of vasomotor symptoms if Black cohosh products approved by regulatory authorities were applied (in contrast to Black cohosh products regulated as food supplementation) [10]. At baseline, menopausal symptoms were moderate to severe in both cohorts and improved over time with the Black cohosh extract Ze 450 and MHT being comparably effective. Interestingly, women with psychological menopausal symptoms were more likely to choose MHT later on. This might be due to the significantly increased risk of depression during perimenopause [20] and the known positive impact of MHT on mood [8, 21]. However, due to the small sample size, we were not able to differentiate between different MHT formulations, dosages, and indications. As significantly more women treated with Ze 450 than MHT were postmenopausal, we can only speculate that MHT was also prescribed for other indications than vasomotor symptom relief, e.g., for menstrual cycle regulation.
Both the menopausal transition and ageing have been shown to be accompanied by body weight increase and adverse changes in body composition and metabolic parameters [1, 2, 4, 5]. A study showed that women with metabolic syndrome had a higher total score of MRS and a higher subscale score for somatic symptoms, such as hot flashes and sweating [22]. For most affected women, any treatment with little adverse effects that may slow down or even stop this progression would be welcomed. For MHT, beneficial effects on body weight, body composition, and metabolic parameters have been described before [6,7,8]. However, many menopausal women and physicians are reluctant to use MHT due to the perceived risks, especially breast cancer risk [23]. Therefore, herbal medicinal products such as Black cohosh or phytoestrogen supplements have gained much attention in recent years. Just recently, a systematic review and meta-analysis reported a decrease in body weight with phytoestrogen supplementation in healthy postmenopausal women, but body weight gain in postmenopausal women with preexisting metabolic disorders [24]. In the present study, we did not observe a negative impact of Ze 450 on body weight gain and metabolic parameters in any subgroup. Yet, in general, women in our cohort were quite healthy. Thus, if a herbal product like Black cohosh dry extract Ze 450 had a stabilizing impact on body weight and metabolic parameters in menopausal women, the net benefit would outperform the rare potential, mostly short-term gastrointestinal, side effects. Our study in women supports previous findings showing a beneficial effect of Ze 450 on AMPK activity in vitro and on metabolic parameters in rats [14]. However, we can only speculate on the exact mechanism of action of Ze 450 in our study as we did not measure AMPK activity.
This is the first study in humans on the impact of Ze 450 on body weight and metabolic parameters. Definitely, these first results need confirmation in bigger and prospective trials.
Conclusion
Both MHT and Black cohosh improved menopausal symptoms. Body weight and serum metabolic parameters did not change in MHT or Black cohosh-treated women.
Data availability
Data were not available as analysis for further publications is in preparation.
References
Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P, Writing Group of the International Menopause Society for World Menopause D (2012) Understanding weight gain at menopause. Climacteric 15(5):419–429
Kapoor E, Collazo-Clavell ML, Faubion SS (2017) Weight gain in women at midlife: a concise review of the pathophysiology and strategies for management. Mayo Clin Proc 92(10):1552–1558
Pimenta F, Maroco J, Ramos C, Leal I (2014) Predictors of weight variation and weight gain in peri- and post-menopausal women. J Health Psychol 19(8):993–1002
Karvonen-Gutierrez C, Kim C (2016) Association of mid-life changes in body size, body composition and obesity status with the menopausal transition. Healthcare (Basel). https://doi.org/10.3390/healthcare4030042
Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA (2009) Lipid changes during the menopause transition in relation to age and weight: the Study of Women’s Health Across the Nation. Am J Epidemiol 169(11):1352–1361
Armeni E, Lambrinoudaki I, Ceausu I, Depypere H, Mueck A, Perez-Lopez FR, Schouw YT, Senturk LM, Simoncini T, Stevenson JC, Stute P, Rees M (2016) Maintaining postreproductive health: a care pathway from the European Menopause and Andropause Society (EMAS). Maturitas 89:63–72
The NAMS 2017 Hormone Therapy Position Statement Advisory Panel (2017) The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 24(7):728–753
Baber RJ, Panay N, Fenton ATIWG (2016) 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 19(2):109–150
The North American Menopause Society (2015) Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 22(11):1155–1174
Beer AM, Osmers R, Schnitker J, Bai W, Mueck AO, Meden H (2013) Efficacy of black cohosh (Cimicifuga racemosa) medicines for treatment of menopausal symptoms—comments on major statements of the Cochrane Collaboration report 2012 “black cohosh (Cimicifuga spp.) for menopausal symptoms (review). Gynecol Endocrinol 29(12):1022–1025
Oktem M, Eroglu D, Karahan HB, Taskintuna N, Kuscu E, Zeyneloglu HB (2007) Black cohosh and fluoxetine in the treatment of postmenopausal symptoms: a prospective, randomized trial. Adv Ther 24(2):448–461
Rhyu MR, Lu J, Webster DE, Fabricant DS, Farnsworth NR, Wang ZJ (2006) Black cohosh (Actaea racemosa, Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human mu opiate receptor. J Agric Food Chem 54(26):9852–9857
Burdette JE, Liu J, Chen SN, Fabricant DS, Piersen CE, Barker EL, Pezzuto JM, Mesecar A, Van Breemen RB, Farnsworth NR, Bolton JL (2003) Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem 51(19):5661–5670
Moser C, Vickers SP, Brammer R, Cheetham SC, Drewe J (2014) Antidiabetic effects of the Cimicifuga racemosa extract Ze 450 in vitro and in vivo in ob/ob mice. Phytomedicine 21(11):1382–1389
Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ, Group SC (2012) Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 19(4):387–395
Heinemann K, Ruebig A, Potthoff P, Schneider HP, Strelow F, Heinemann LA, Do MT (2004) The Menopause Rating Scale (MRS) scale: a methodological review. Health Quality Life Outcomes 2:45
Bischoff-Ferrari HA, Rosemann T, Grob D, Theiler R, Simmen H-P, Meyer O (2014) Vitamin-D-supplementation in der Praxis. Schweiz Med Forum 14(50):949–953
Krul-Poel YH, Ter Wee MM, Lips P, Simsek S (2017) Management of endocrine disease: the effect of vitamin D supplementation on glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Eur J Endocrinol 176(1):R1–R14
Schellenberg R, Saller R, Hess L, Melzer J, Zimmermann C, Drewe J, Zahner C (2012) Dose-dependent effects of the Cimicifuga racemosa extract Ze 450 in the treatment of climacteric complaints: a randomized, placebo-controlled study. Evid Based Complement Altern Med 2012:260301
Freeman EW, Sammel MD, Lin H, Nelson DB (2006) Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 63(4):375–382
MacQueen GM, Frey BN, Ismail Z, Jaworska N, Steiner M, Lieshout RJ, Kennedy SH, Lam RW, Milev RV, Parikh SV, Ravindran AV, Group CDW (2016) Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 6. Special populations: youth, women, and the elderly. Can J Psychiatry 61(9):588–603
Lee SW, Jo HH, Kim MR, Kwon DJ, You YO, Kim JH (2012) Association between menopausal symptoms and metabolic syndrome in postmenopausal women. Arch Gynecol Obstet 285(2):541–548
Yeganeh L, Boyle J, Teede H, Vincent A (2017) Knowledge and attitudes of health professionals regarding menopausal hormone therapies. Climacteric 20(4):348–355
Glisic M, Kastrati N, Musa J, Milic J, Asllanaj E, Portilla Fernandez E, Nano J, Ochoa Rosales C, Amiri M, Kraja B, Bano A, Bramer WM, Roks AJM, Danser AHJ, Franco OH, Muka T (2018) Phytoestrogen supplementation and body composition in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Maturitas 115:74–83
Acknowledgements
We would like to extend special acknowledgements to Andrea Isler for data extraction. Max Zeller Söhne AG, Romanshorn, Switzerland, supported this work.
Author information
Authors and Affiliations
Contributions
FL: data collection and manuscript editing. NS: manuscript writing and editing, and project administration. ZC: project development, and manuscript writing and editing. BL: data management, data analysis, and manuscript writing and editing. SP: project development, investigation, methodology, manuscript writing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
This is an investigator-initiated monocentric, retrospective, observational study. The study was supported by Max Zeller Söhne AG, Romanshorn. Catherine Zahner and Sabine Nebel are employees of Max Zeller Söhne AG. The authors were autonomous in data analyses and study report and declare no conflict of interest.
Informed consent
Informed consent is not available as data are used retrospectively which have been collected already and it is impossible or disproportionately difficult to obtain consent due to the high number of patients.
Ethical approval
In addition, documented refusal is not available in any of the patient files (as per clinical trial protocol approved by the cantonal ethics committee No 2016-01179).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file 1
Overview of the assessed blood chemistry, personal and family history. (DOCX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Friederichsen, L., Nebel, S., Zahner, C. et al. Effect of CIMicifuga racemosa on metaBOLIC parameters in women with menopausal symptoms: a retrospective observational study (CIMBOLIC). Arch Gynecol Obstet 301, 517–523 (2020). https://doi.org/10.1007/s00404-019-05366-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05366-8