Abstract
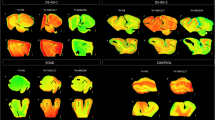
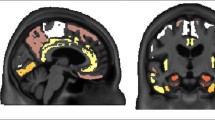
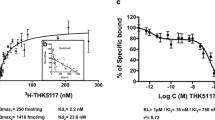
[F-18]-AV-1451, a PET tracer specifically developed to detect brain neurofibrillary tau pathology, has the potential to facilitate accurate diagnosis of Alzheimer’s disease (AD), staging of brain tau burden and monitoring disease progression. Recent PET studies show that patients with mild cognitive impairment and AD dementia exhibit significantly higher in vivo [F-18]-AV-1451 retention than cognitively normal controls. Importantly, PET patterns of [F-18]-AV-1451 correlate well with disease severity and seem to match the predicted topographic Braak staging of neurofibrillary tangles (NFTs) in AD, although this awaits confirmation. We studied the correlation of autoradiographic binding patterns of [F-18]-AV-1451 and the stereotypical spatiotemporal pattern of progression of NFTs using legacy postmortem brain samples representing different Braak NFT stages (I-VI). We performed [F-18]-AV-1451 phosphor-screen autoradiography and quantitative tau measurements (stereologically based NFT counts and biochemical analysis of tau pathology) in three brain regions (entorhinal cortex, superior temporal sulcus and visual cortex) in a total of 22 cases: low Braak (I–II, n = 6), intermediate Braak (III–IV, n = 7) and high Braak (V–VI, n = 9). Strong and selective [F-18]-AV-1451 binding was detected in all tangle-containing regions matching precisely the observed pattern of PHF-tau immunostaining across the different Braak stages. As expected, no signal was detected in the white matter or other non-tangle containing regions. Quantification of [F-18]-AV-1451 binding was very significantly correlated with the number of NFTs present in each brain region and with the total tau and phospho-tau content as reported by Western blot and ELISA. [F-18]-AV-1451 is a promising biomarker for in vivo quantification of brain tau burden in AD. Neuroimaging–pathologic studies conducted on postmortem material from individuals imaged while alive are now needed to confirm these observations.



Similar content being viewed by others
References
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. doi:10.1007/s00401-006-0127-z
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab Jet al (2016) Tau and Abeta imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med 8:338ra366. doi:10.1126/scitranslmed.aaf2362
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL 3rd, Schneider JA, Grinberg LT, Halliday G et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22. doi:10.1007/s00401-007-0237-2
Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, Shankle WR, Elizarov A, Kolb HC (2013) Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimer’s Dis JAD 34:457–468. doi:10.3233/JAD-122059
Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, Lee JH, Ryu YH, Lee MS, Lyoo CH (2016) In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol 80:247–258. doi:10.1002/ana.24711
Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, Lyoo CH, Ryu YH, Lee MS (2016) Tau PET in Alzheimer disease and mild cognitive impairment. Neurology 87:375–383. doi:10.1212/WNL.0000000000002892
Gordon BA, Friedrichsen K, Brier M, Blazey T, Su Y, Christensen J, Aldea P, McConathy J, Holtzman DM, Cairns NJ et al (2016) The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain J Neurol 139:2249–2260. doi:10.1093/brain/aww139
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement J Alzheimer’s Assoc 8:1–13. doi:10.1016/j.jalz.2011.10.007
Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K et al (2016) Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 79:110–119. doi:10.1002/ana.24546
Josephs KA, Whitwell JL, Tacik P, Duffy JR, Senjem ML, Tosakulwong N, Jack CR, Lowe V, Dickson DW, Murray ME (2016) [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol 132:931–933. doi:10.1007/s00401-016-1618-1
Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, Kantarci K, Boeve BF, Pandey MK, Bruinsma T et al (2016) An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 4:58. doi:10.1186/s40478-016-0315-6
Marquie M, Normandin MD, Meltzer AC, Siao Tick Chong M, Andrea NV, Anton-Fernandez A, Klunk WE, Mathis CA, Ikonomovic MD, Debnath M et al (2017) Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol 81:117–128. doi:10.1002/ana.24844
Marquie M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, Klunk WE, Mathis CA, Ikonomovic MD, Debnath ML et al (2015) Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 78:787–800. doi:10.1002/ana.24517
Mattsson N, Rosen E, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M et al (2012) Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology 78:468–476. doi:10.1212/WNL.0b013e3182477eed
McKeith IG (2006) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimer’s Dis JAD 9:417–423
McMillan CT, Irwin DJ, Nasrallah I, Phillips JS, Spindler M, Rascovsky K, Ternes K, Jester C, Wolk DA, Kwong LK et al (2016) Multimodal evaluation demonstrates in vivo 18F-AV-1451 uptake in autopsy-confirmed corticobasal degeneration. Acta Neuropathol 132:935–937. doi:10.1007/s00401-016-1640-3
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K et al (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71:362–381. doi:10.1097/NEN.0b013e31825018f7
Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, Fernandez-Carballo L, de Munain EL, Perez J, Marquie M et al (2013) Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain J Neurol 136:2510–2526. doi:10.1093/brain/awt171
Pontecorvo MJ, Devous MD Sr, Navitsky M, Lu M, Salloway S, Schaerf FW, Jennings D, Arora AK, McGeehan A, Lim NC et al (2017) Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain J Neurol. doi:10.1093/brain/aww334
Qian J, Hyman BT, Betensky RA (2017) Neurofibrillary tangle stage and the rate of progression of Alzheimer symptoms: modeling using an autopsy cohort and application to clinical trial design. JAMA Neurol. doi:10.1001/jamaneurol.2016.5953
Riley KP, Snowdon DA, Markesbery WR (2002) Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol 51:567–577. doi:10.1002/ana.10161
Rosen C, Hansson O, Blennow K, Zetterberg H (2013) Fluid biomarkers in Alzheimer’s disease—current concepts. Mol Neurodegener 8:20. doi:10.1186/1750-1326-8-20
Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D et al (2007) Imaging beta-amyloid burden in aging and dementia. Neurology 68:1718–1725. doi:10.1212/01.wnl.0000261919.22630.ea
Sander K, Lashley T, Gami P, Gendron T, Lythgoe MF, Rohrer JD, Schott JM, Revesz T, Fox NC, Arstad E (2016) Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimer’s Dement J Alzheimer’s Assoc 12:1116–1124. doi:10.1016/j.jalz.2016.01.003
Scholl M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD et al (2016) PET imaging of tau deposition in the aging human brain. Neuron 89:971–982. doi:10.1016/j.neuron.2016.01.028
Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, Joshi AD, Devous MD Sr, Mintun MS (2016) Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain J Neurol. doi:10.1093/brain/aww023
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189. doi:10.1101/cshperspect.a006189
Smith R, Scholl M, Honer M, Nilsson CF, Englund E, Hansson O (2017) Tau neuropathology correlates with FDG-PET, but not AV-1451-PET, in progressive supranuclear palsy. Acta Neuropathol 133:149–151. doi:10.1007/s00401-016-1650-1
Villemagne VL, Ong K, Mulligan RS, Holl G, Pejoska S, Jones G, O’Keefe G, Ackerman U, Tochon-Danguy H, Chan JG et al (2011) Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J Nucl Med Off Publ Soc Nucl Med 52:1210–1217. doi:10.2967/jnumed.111.089730
Vonsattel JP, Del Amaya MP, Keller CE (2008) Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol 115:509–532. doi:10.1007/s00401-007-0311-9
Wang L, Benzinger TL, Su Y, Christensen J, Friedrichsen K, Aldea P, McConathy J, Cairns NJ, Fagan AM, Morris JC et al (2016) Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between beta-amyloid and tauopathy. JAMA Neurol. doi:10.1001/jamaneurol.2016.2078
Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, Lam C, Liang Q, Liu C, Mocharla VP et al (2013) [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimer’s Dement J the Alzheimer’s Assoc 9:666–676. doi:10.1016/j.jalz.2012.11.008
Acknowledgements
We are grateful to all the study subjects who donated their brains to the MADRC Neuropathology Core, the MGH PET Core for providing [F-18]-AV-1451 and Dr. Peter Davies, from the Feinstein Institute for Medical Research, for kindly sharing the PHF-1 antibody.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Marta Marquié received research funding from the ASISA Foundation in Madrid, Spain. Alejandro Antón-Fernández received funding from the Ministry of Economy and Competitiveness-State Secretariat for Research, Development and Innovation of Spain (SAF 2015-66603-P). Eline E. Verwer received support from the Society of Nuclear Medicine and Molecular Imaging Education and Research Foundation. Marc D. Normandin received research funding from NIH National Institute of Neurological Disorders and Stroke (U01NS086659) and NIH National Institute of Mental Health (R01MH100350). Matthew P. Frosch received research funding from the Massahcusetts Alzheimer’s Disease Research Center. Teresa Gómez-Isla received research funding from NIH National Institute on Aging (AG005134 and AG036694).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Marquié, M., Siao Tick Chong, M., Antón-Fernández, A. et al. [F-18]-AV-1451 binding correlates with postmortem neurofibrillary tangle Braak staging. Acta Neuropathol 134, 619–628 (2017). https://doi.org/10.1007/s00401-017-1740-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-017-1740-8