Abstract
For the improvement of poly (N-isopropylacrylamide) properties, new series of poly (N-isopropylacrylamide-Co-acrylic acid-Co-vanillin acrylate) terpolymer was fabricated with molar concentrations 5, 10, and 15 mol% of acrylic acid (AA) with 10 mol% of vanillin acrylate (VA). Monomer and polymers were chemically evaluated using (1H NMR, 13C, FT-IR, and UV); all structures emphasized logical results. The post-polymerization has been done for terpolymer with tryptophan and threonine; they were chemically investigated. Polymers and post-polymers have been physically characterized using SEM, X-ray, DSC, TGA, and SEM. Lower critical solution temperature was measured using turbidity by UV–VIS spectroscopy and by micro-DSC. These polymers will be used in the post-polymerization of many biomolecules for biomedical applications
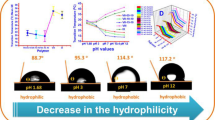
Graphical abstract














Similar content being viewed by others
References
Abdelaty MSA (2021) Trends in the phase separation temperature optimization of a functional and thermo-pH responsive terpolymer of poly (N-isopropylacrylamide-co-N-(2-(dimethylamino)ethyl) acrylamide-co-vanillin acrylate). J Polym Environ. https://doi.org/10.1007/s10924-021-02096-4
Liang H, Qiang Z, Xue L, Michael JS (2019) Stimuli-responsive polymers for sensing and actuation. Mater Horiz 6:1774–1793. https://doi.org/10.1039/C9MH00490D
Seidi F, Jenjob R, Crespy D (2018) Designing smart polymer conjugates for controlled release of payloads. Chem Rev 11:3965–4036. https://doi.org/10.1021/acs.chemrev.8b00006
Xiaoming H, Chen Z, Yufu T, Feng L, Yuanyuan L, Feng P, Xiaomei L, Yu J, Jie L, Wenjun W, Quli F, Wei H (2019) Intelligent polymer–MnO2 nanoparticles for dual-activatable photoacoustic and magnetic resonance bimodal imaging in living mice. Chem Commun 55:6006–6009. https://doi.org/10.1039/C9CC02148E
Abdelaty MSA (2018) Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4 formyl-6-methoxyphenylacrylate) environmental functional copolymers: synthesis, characterizations, and grafting with amino acids. Biomolecules 8:138. https://doi.org/10.3390/biom8040138
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin. J Polym Environ 26:2243–2256. https://doi.org/10.1007/s10924-017-1126-y
Abdelaty MSA (2018) Preparation and characterization of new environmental functional polymers based on vanillin and N-isopropylacrylamide for post polymerization. J Polym Environ 26:636–646. https://doi.org/10.1007/s10924-017-0960-2
Chen J-K, Chang C-J (2014) Fabrication and applications of stimuli-responsive polymer films and patterns on surface. Materials 7:805–875. https://doi.org/10.3390/ma7020805
Richard H (2014) Temperature-responsive polymers: properties, synthesis, and applications, chapter 2, 13–44. https://doi.org/10.1016/B978-0-08-102416-4.00002-8
Kocak G, Tuncer C, Bütün V (2017) pH-Responsive polymers. Polym Chem 8:144–176. https://doi.org/10.1039/C6PY01872F
Tao X, Ting L, Wei-Feng Z, Cheng-Sheng Z (2019) Ionic-strength responsive Zwitterionic copolymer hydrogels with tunable swelling and adsorption behaviors. Langmuir 35(5):1146–1155
Shohei I, Miki M, Hironobu K, Yoshitsugu H, Shokyoku K (2019) Swelling and mechanical properties of thermo-responsive/hydrophilic conetworks with crosslinked domain structures prepared from various triblock precursors. Polym Chem Advance Article. https://doi.org/10.1039/C9PY01417A
Valentina M, Pierfrancesco C, Marta G, Bartosz T, Veronica A (2017) Light-responsive polymer micro- and nano-capsules. Polymers 9:1–19. https://doi.org/10.3390/polym9010008
Chikara K, Akihiro K, Kenji U, Toshikazu T, Masatoshi K, Kohzo I (2013) Pressure-responsive polymer membranes of slide-ring gels with movable cross-links. Advanced material 6:4636–4640. https://doi.org/10.1002/adma.201301252
Gil ES, Hudson SM (2004) Stimuli-responsive polymers and their bioconjugates. Prog Polym Sci 29:1173–1222. https://doi.org/10.1016/j.progpolymsci.2004.08.003
Shibayama M, Tanaka T (1993) Volume phase transition and related phenomena of polymer gels. Adv Polym Sci 109:1–62. https://doi.org/10.1007/3-540-56791-7-1
Chen G, Hoffman AS (1995) Graft copolymers that exhibit temperature-induced phase transition over a wide range of pH. Nature 373:49–52. https://doi.org/10.1038/373049a0
Hoffman AS, Stayton PS, Bulmus V (2000) Really smart bioconjugates of smart polymers and receptor proteins). J Biomed Mater Res 52:577–586. https://doi.org/10.1002/1097-4636(20001215)52
Costa E, Coelho M, Ilharco LM, Aguiar-Ricardo A, Hammond PT (2011) Tannic acid mediated suppression of PNIPAAm microgels thermoresponsive behaviour. Macromolecules 44:612–621. https://doi.org/10.1021/ma1025016
Yang HW, Chena JK, Cheng CC, Kuo SW (2013) Association of poly(N-isopropylacrylamide) containing nucleobase multiple hydrogen bonding of adenine for DNA recognition. Appl Surf Sci 271:60–69. https://doi.org/10.1016/j.apsusc.2013.01.074
Jan S, Seema A (2013) Polymers with upper critical solution temperature in aqueous solution: unexpected properties from known building blocks. ACS Macro Lett 7:597–600. https://doi.org/10.1021/mz400227y
Sonia L, Elaine A (2017) Poly(N-isopropylacrylamide) and copolymers: a review on recent progresses in biomedical applications. Gels 3:1–32. https://doi.org/10.3390/gels3040036
Kocak G, Tuncera C, Bütün V (2017) pH-Responsive polymers. Polym Chem 8:144–176. https://doi.org/10.1039/C6PY01872F
Katchalsky A, Eisenberg H (1951) J Polym Sci 6:145–154
Thomas S, Linda S, Mark G, Stephen R (2016) The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 12:2542. https://doi.org/10.1039/c5sm02693h
Chen D, Liu H, Kobayashib T, Yu H (2010) Multiresponsive reversible gels based on a carboxylic azo polymer. J Mater Chem 20:3610–3614. https://doi.org/10.1039/B925163D
Pasparakisa G, Vamvakaki M (2011) Multiresponsive polymers: nano-sized assemblies, stimuli-sensitive gels and smart surfaces. Polym Chem 2:1234
George P, Maria V (2011) Multiresponsive polymers: nano-sized assemblies, stimuli-sensitive gels and smart surfaces. Polym Chem 2:1234–1248
Yajie L, Chaocan Z, Youliang Z, Yixiao D, Wanyu C (2015) Novel multi-responsive polymer materials: when ionic liquids step in. Euro polym j 69:441–448. https://doi.org/10.1016/j.eurpolymj.2015.05.023
Sebastian H, Torsten R, Hendrik B, Sebastian S (2014) Multiresponsive polymer hydrogels by orthogonal supramolecular chain cross-linking. Macromolecules 47(12):4028–4036. https://doi.org/10.1021/ma5008573
Hossein H, Soleyman H, Shahryar P, Naser G, Rohollah M (2019) Synthesis of multiresponsive β-cyclodextrin nanocomposite through surface RAFT polymerization for controlled drug delivery. Polymers advanced technologies 30:2860–2871. https://doi.org/10.1002/pat.4718
Ramkissoon-Ganorkar C, Baudys M, Wan Kim S (2000) Effect of ionic strength on the loading efficiency” of the model polypeptide/protein drugs in pH-/temperature-sensitive polymers. J Biomat Sci Polym Ed 11:45–54 (PMID: 10680607)
Ju HK, Kim SY, Kim SJ, Lee YM (2002) pH/temperature-responsive semi-IPN hydrogels composed of alginate and poly(N-isopropylacrylamide. J Appl Polym Sci 11:28–1139. https://doi.org/10.1002/app.10137
Abdelaty MSA (2021) Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4-methylphenyl acrylate) thermo-ph responsive copolymer: trend in the lower critical solution temperature optimization of Poly (N-isopropyylacrylamide). J Polym Res 28:213. https://doi.org/10.1007/s10965-021-02574-2
Abdelaty MSA (2019) Influence of vanillin acrylate and 4-acetylphenyl acrylate hydrophobic functional monomers on phase separation of N-isopropylacrylamide environmental terpolymer: fabrication and characterization. Polym Bull. https://doi.org/10.1007/s00289-019-02890-0
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin (part 2): temperature responsive layer A, functional, temperature and ph layer B. Polym Bull 11:4837–4858. https://doi.org/10.1007/s00289-018-2297-y
Abdelaty MSA (2018) Preparation and functional, temperature and ph layer poly(styrene-Co-2-[(diethylamino)methyl]-4-formyl-6-methoxy-phenyl acrylate) copolymers for amino acid post polymerization. Open J Org Polym Mater 8:41–55. https://doi.org/10.4236/ojpchem.2018.83005
Abdelaty MSA, Kuckling D (2018) Poly (N-isopropyl acrylamide-Co-vanillin acrylate) dual responsive functional copolymers for grafting biomolecules by Schiff’s base click reaction. Open J Org Polym Mater 8:15–32. https://doi.org/10.4236/ojopm.2018.82002
Abdelaty, MSA (2019) Layer by layer photo-cross-linked environmental functional hydrogel thin films based on vanillin: part 3. J Polym Environ. https://doi.org/10.1007/s10924-019-01421-2
Abdelaty MSA, Kuckling D (2016) Synthesis and characterization of new functional photo cross-linkable smart polymers containing vanillin derivatives. Gels 2:1–13. https://doi.org/10.3390/gels2010003
Yeong-Tarng S, Pei-Yi L, Shiao-Wei K (2018) Sequence length distribution affects the lower critical solution temperature, glass transition temperature, and CO2-responsiveness of N-isopropylacrylamide/methacrylic acid copolymers. Polymer 143:258–270. https://doi.org/10.1016/j.polymer.2018.04.002
Yeong-Tarng S, Bo-Hong C, Tzong-Liu W, Shiao-Wei K (2017) Supercritical CO2 affects the copolymerization, LCST behavior, thermal properties, and hydrogen bonding interactions of poly(N-isopropylacrylamide-co-acrylic acid). The Journal of Supercritical Fluids 130:373–380. https://doi.org/10.1016/j.supflu.2017.07.004
Yeong-Tarng S, Pei-Yi L, Tao, C,; Shiao-Wei, K, (2016) Temperature-, pH- and CO2-sensitive poly(N-isopropylacryl amide-co-acrylic acid) copolymers with high glass transition temperatures. Polymers 8(12):434. https://doi.org/10.3390/polym8120434
Abdelaty MSA (2021) A facile method for the preparation of hydrophilic-hydrophobic functional thermo-pH responsive terpolymers based on poly(NIPAAm-co-DMAA-co-DMAMVA) and post-polymerization J Polym Environ. https://doi.org/10.1007/s10924-021-02117-2
Aditya J, Nandi D, Chester A, Marie M (2018) Study of Poly(N-isopropylacrylamide-co-acrylic acid) (pNIPAM) microgel particle induced deformations of tissue-mimicking phantom by ultrasound stimulation. Langmuir 34:1457–1465. https://doi.org/10.1021/acs.langmuir.7b02801
Fache M, Darroman E, Besse V, Auvergne R, Sylvain Caillol S, Boutevina., B. (2014) Vanillin, a promising biobased building-block for monomer synthesis. Green Chem 16:1987–1998. https://doi.org/10.1039/C3GC42613K
Ananda SA, Bernard W, Ashfaqur R (2012) Vanillin based polymers: I. An electrochemical route to polyvanillin. Green Chem 14:2395–2397. https://doi.org/10.1039/C2GC35645G
Firdaus MM, Meier AR (2013) Renewable copolymers derived from vanillin and fatty acid derivatives. Eur Polym J 49:156–166. https://doi.org/10.1016/j.eurpolymj.2012.10.017
Abdelaty MSA, (2019) Layer by layer photo-cross-linked environmental functional hydrogel thin films based on vanillin: part 3. J Polym Environ 3. https://doi.org/10.1007/s10924-019-01421-2
Sun H, Gao C (2010) Facile synthesis of multiamino vinyl poly (amino acid)s for promising bioapplications. Biomacromol 11:3609–3616. https://doi.org/10.1021/bm101060m
Bauri K, Roy SG, Pant S, De P (2013) Controlled synthesis of amino acid-based pH-responsive chiral polymers and self-assembly of their block copolymers. Langmuir 29:2764–2774. https://doi.org/10.1021/la304918s
Saswati GR, Priyadarsi D (2014) pH responsive polymers with amino acids in the side chains and their potential applications. J Appl Polym Sci 41084:1–12. https://doi.org/10.1002/APP.410
Vincent L, Alexandre C, Mona S, Steven PA (2013) Synthesis and characterization of poly(amino acid)-stabilized diblock copolymer nano-objects. J Name 00:1–3. https://doi.org/10.1039/x0xx00000x
Hideharu M, Ikumi K, Shoko S, Takeshi E (2010) Proline-based block copolymers displaying upper and lower critical solution temperatures. Macromolecules 43:1289–1298. https://doi.org/10.1021/ma902002b
Shimazaki Y, Takani M, Yamauchi O (2009) Metal complexes of amino acids and amino acid side chain groups Structures and properties. Dalton Trans 14:7854–7869. https://doi.org/10.1039/b905871k7854
Qiu Y, Park K (2001) Environment-sensitive hydrogels for drug delivery. Adv Drug Deliver Rev 53:321–339. PII: S0169–409X(01)00203–4
Bauri K, Nandi M, De P (2018) Amino acid-derived stimuli-responsive polymers and their applications. Polym Chem 9:1257–1287. https://doi.org/10.1039/C7PY02014G
Swagatam B, Mohini MK, Sandip S, Jayanta H (2019) Amino acid conjugated polymers: antibacterial agents effective against drug-resistant Acinetobacter baumannii with no detectable resistance. ACS Appl Mater Interfaces 11(37):33559–33572. https://doi.org/10.1021/acsami.9b09016
Xin Y, Yuan J (2012) Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym Chem 3:3045–3055. https://doi.org/10.1002/pc.22327
McKay CS, Finn MG (2014) Click chemistry in complex mixtures: bioorthogonal bioconjugation. Chem Biol 21:1075–1101. https://doi.org/10.1016/j.chembiol.2014.09.002
Junpeng X, Yi L, Shan-hui H (2019) Hydrogels based on Schiff base linkages for biomedical applications. Molecules 24:3005. https://doi.org/10.3390/molecules24163005
Trask RS, Williams HR, Bond IP (2007) Self-healing polymer composites: mimicking nature to enhance performance. Bioinspir Biomim 2:1–9. https://doi.org/10.1088/1748-3182/2/1/P01
Zhang Z, He C, Chen X (2018) Hydrogels based on pH-responsive reversible carbon–nitrogen double-bond linkages for biomedical applications. Mater Chem Front 2:1765–1778. https://doi.org/10.1039/C8QM00317C
Huang J, Deng Y, Ren J, Chen G, Wang G, Wang F, Wu X (2018) Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr Polym 186:54–63. https://doi.org/10.1016/j.carbpol.2018.01.025
Zhao X, Wu H, Guo BL, Dong RN, Qiu YS, Ma PX (2017) Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 122:34–47. https://doi.org/10.1016/j.biomaterials.2017.01.011
Acknowledgements
We would like to thank the Paderborn University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelaty, M.S.A., Kuckling, D. Altering of lower critical solution temperature of environmentally responsive poly (N-isopropylacrylamide-co-acrylic acid-co-vanillin acrylate) affected by acrylic acid, vanillin acrylate, and post-polymerization modification. Colloid Polym Sci 299, 1617–1629 (2021). https://doi.org/10.1007/s00396-021-04882-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-021-04882-x